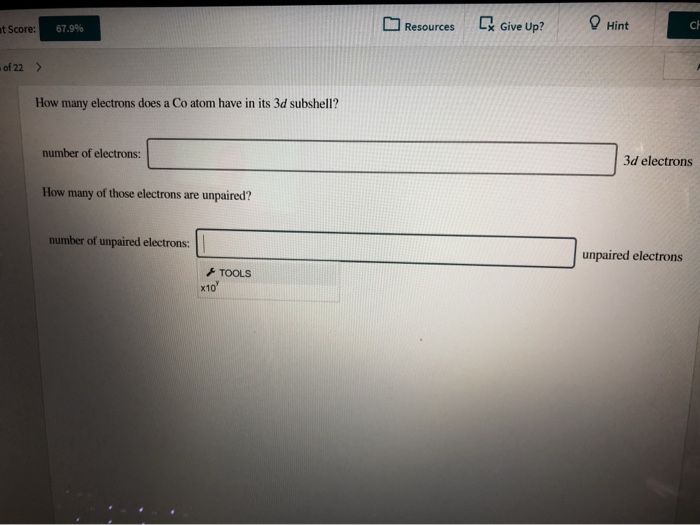
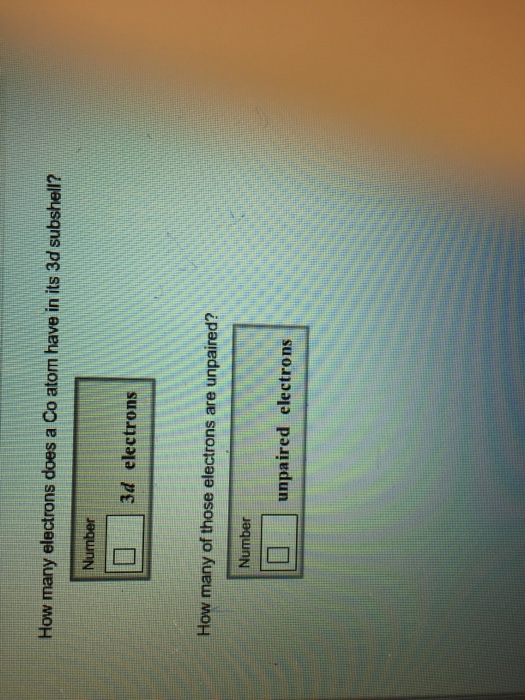
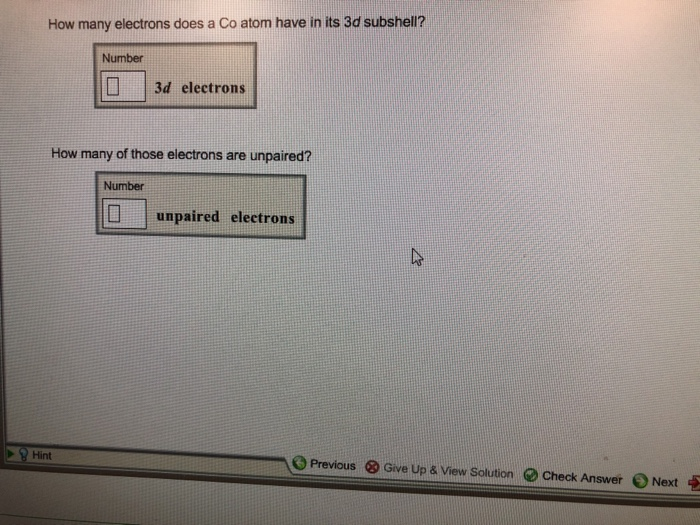
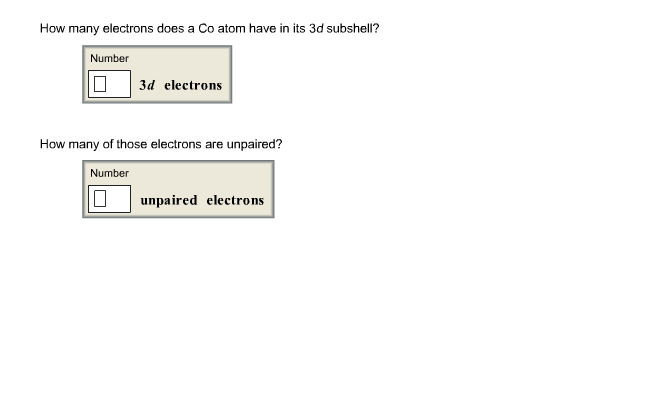
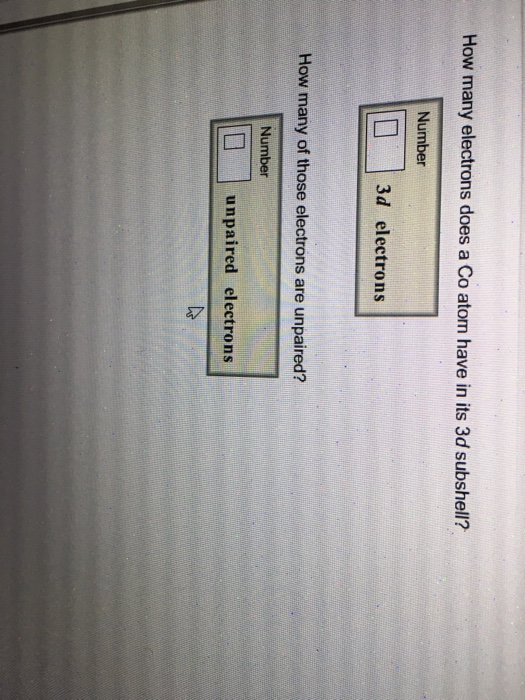
How Many Electrons Does A Co Atom Have In Its 3d Subshell
How many of those electrons are unpaired.

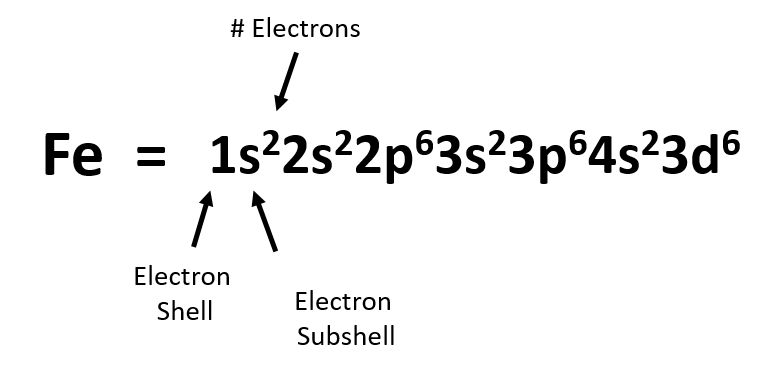
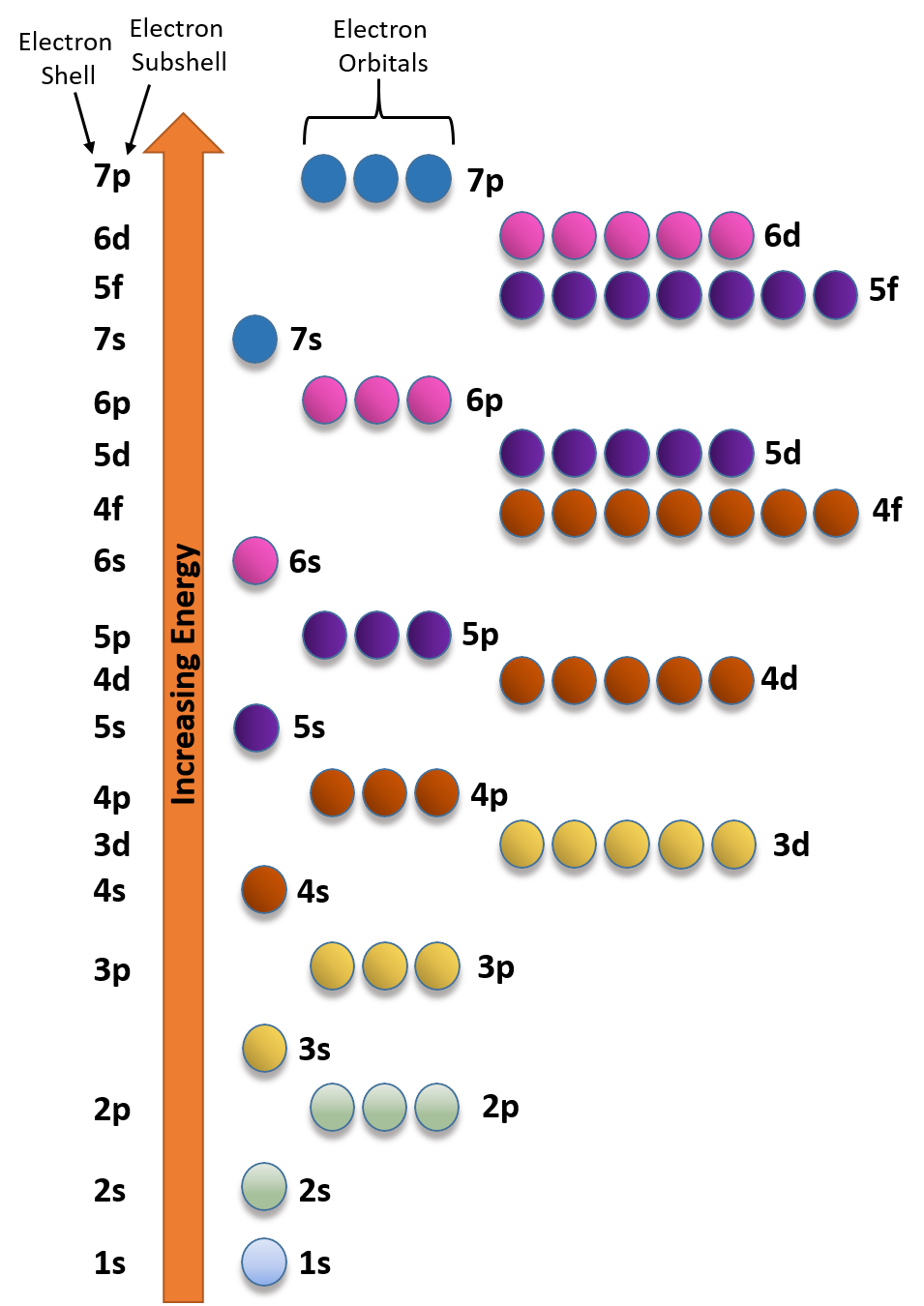
How many electrons does a co atom have in its 3d subshell. Atomic number 27 of electrons 27 e. Answer to how many electrons does a co atom have in its 3d subshell. How many electrons does a co atom have in its 3d subshell. How many of those electrons are unpaired.
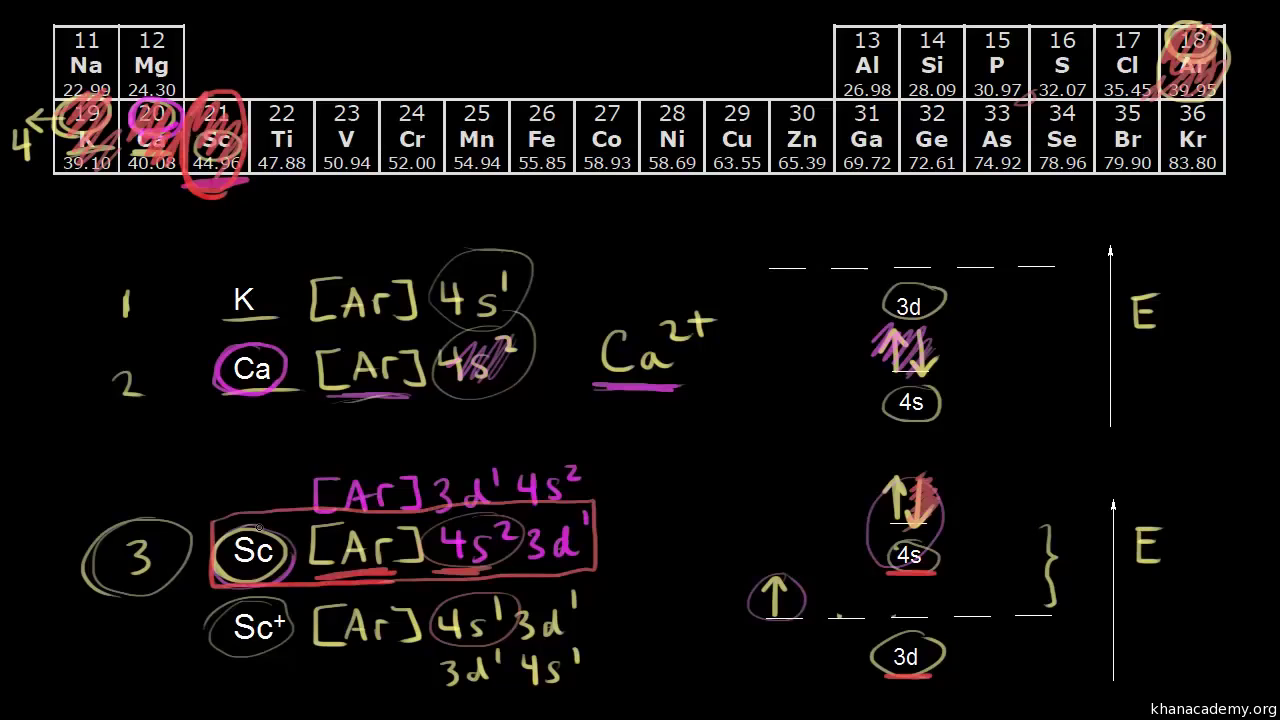
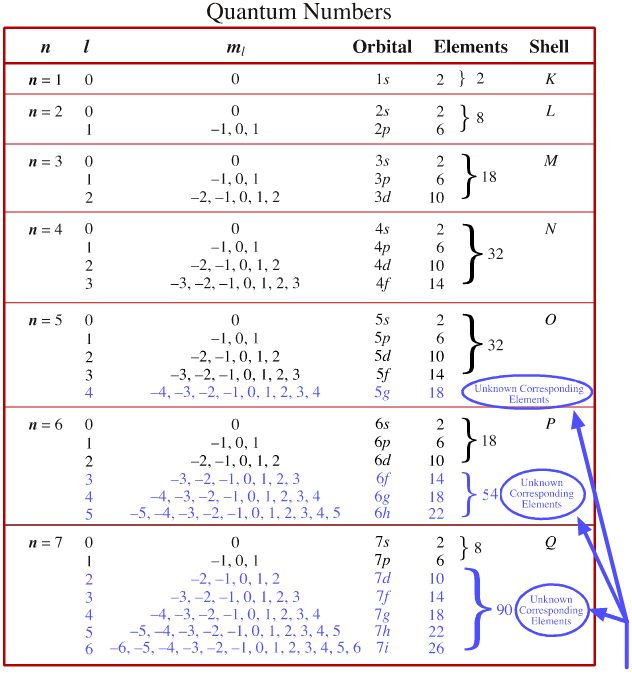
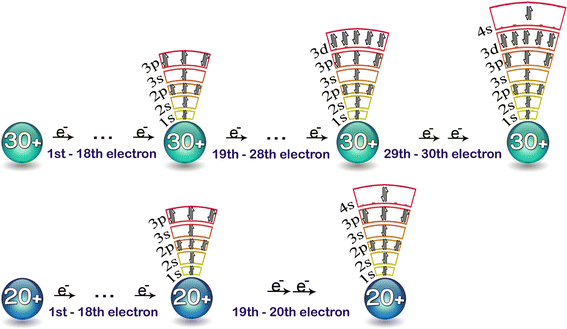
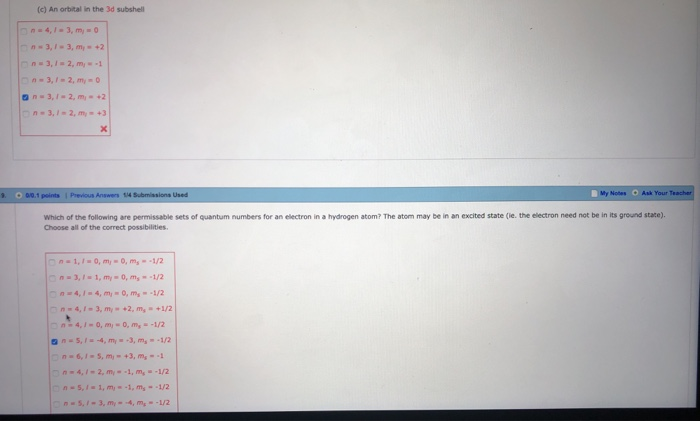
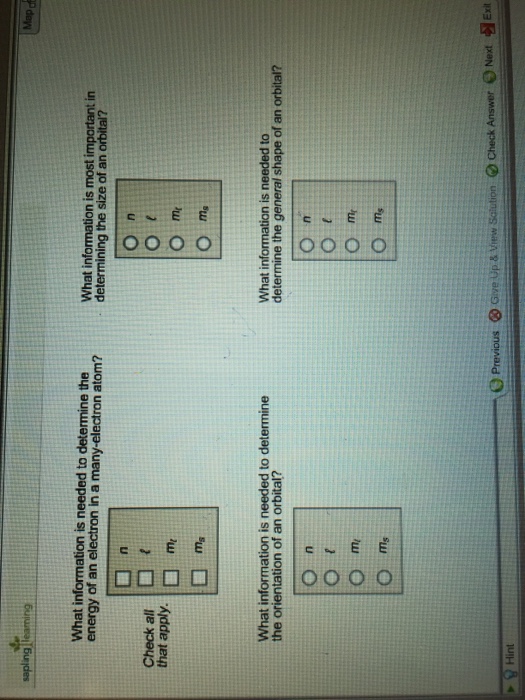
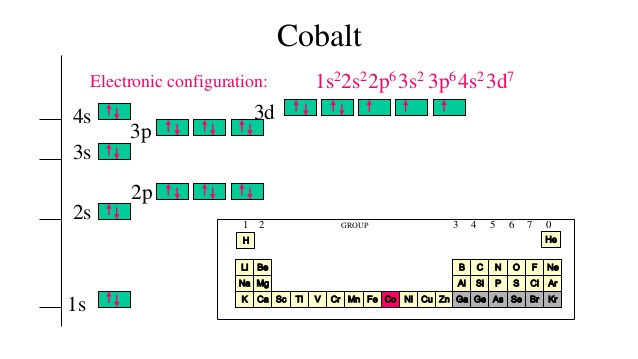
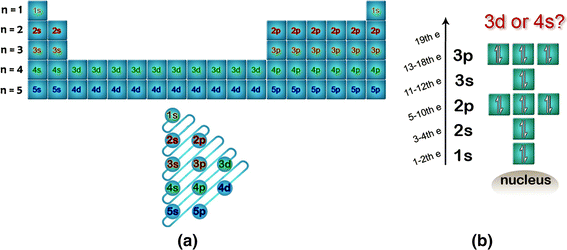
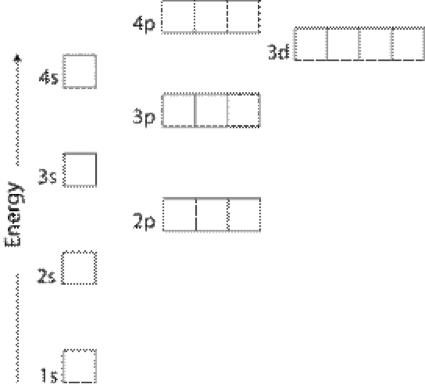
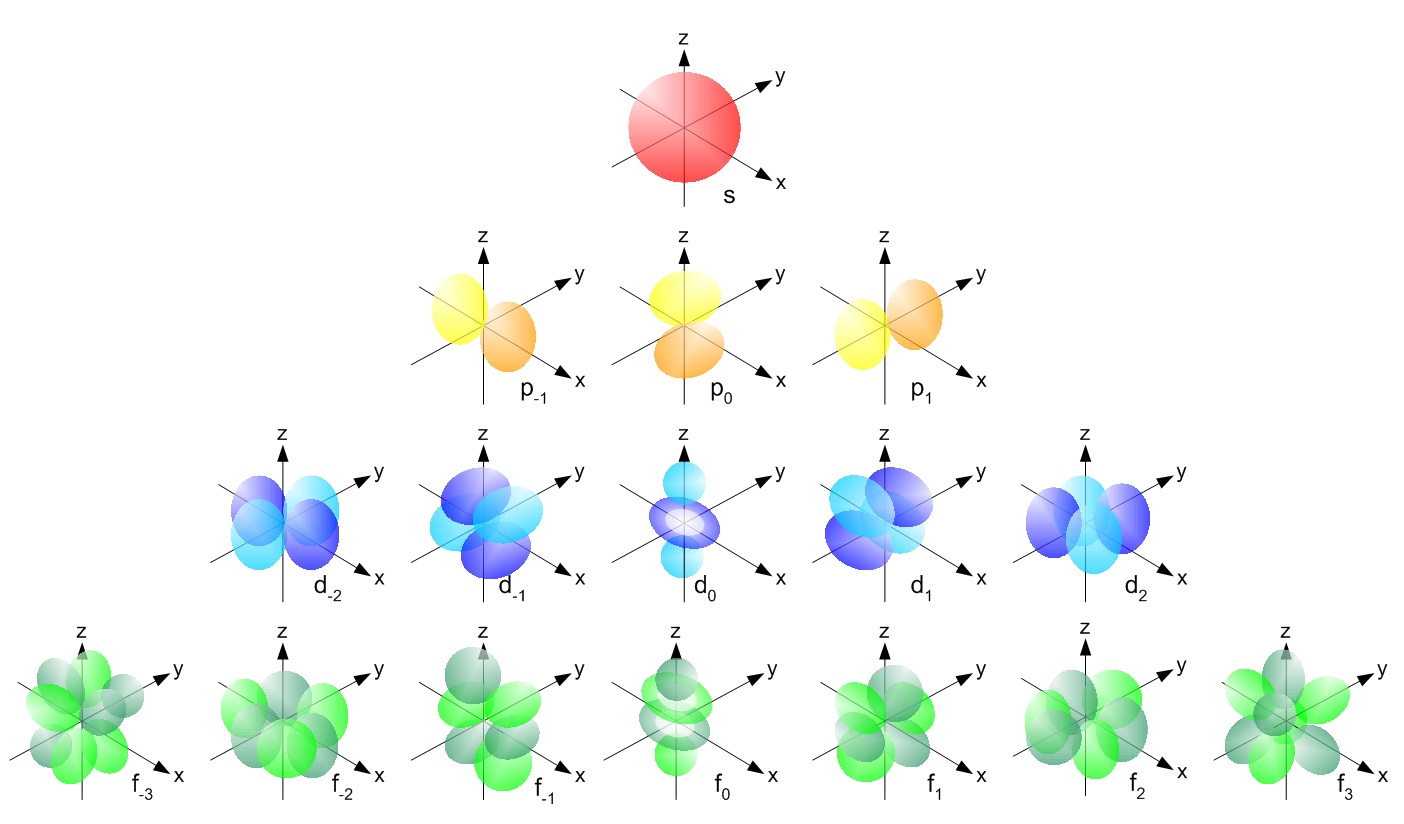
Lets first write the electron configuration of co atom. Which of the following is a possible set of quantum numbers to describe an electron in a 3d subshell. Atomic number protons of electrons. Answer to how many electrons does a co atom have in its 3d subshell.
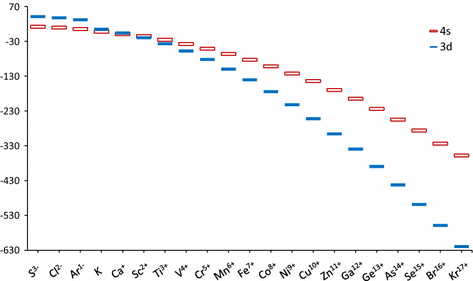
How many core electrons does a chlorine atom have. Since the 3s subshell can hold 2 the 3p can hold 6 and the 3d can hold 10 the third energy level can hold 2 6 10 18 electronsit is important to note however that when filling the third. Unpaired electrons get more help from chegg. How many electrons does a co atom have in its 3d subshell.
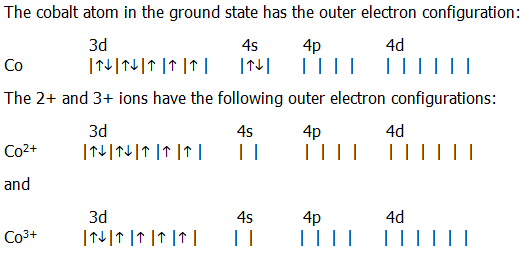
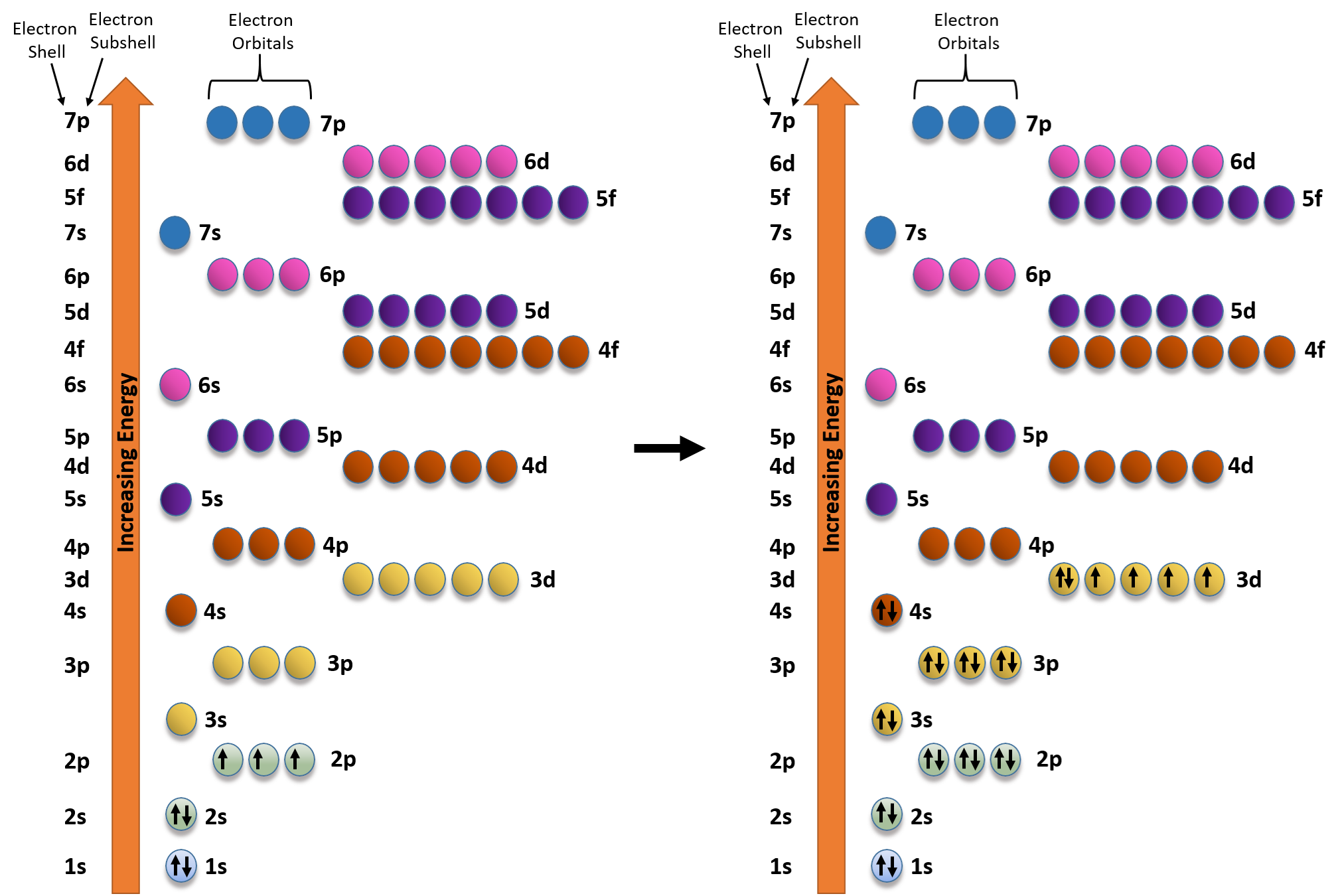
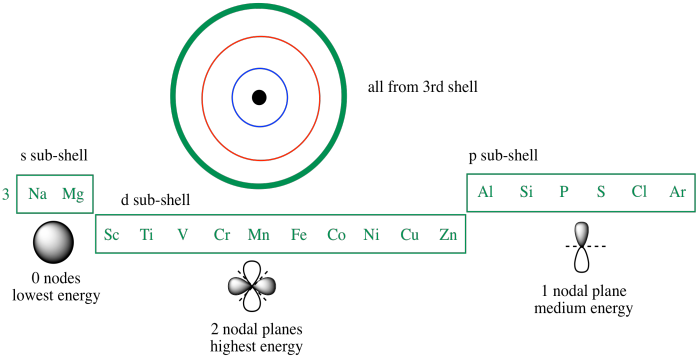
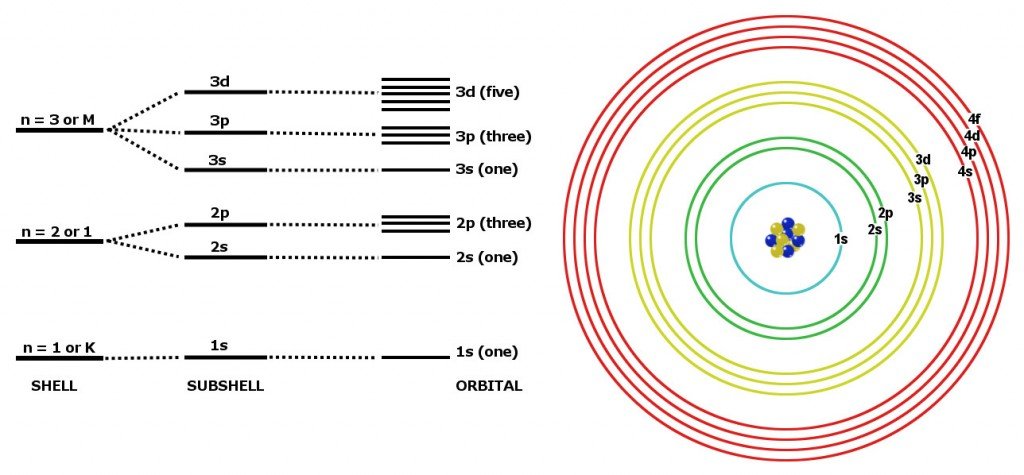
Expert answer 100 54 ratings previous question next question. Cobalt has 7 electrons in the 3d subshell and 3 of them are unpaired. Hhhow many electrons are in the 4p subshell of selenium. 1st shell has s orbital and can hold up to 2 electrons.
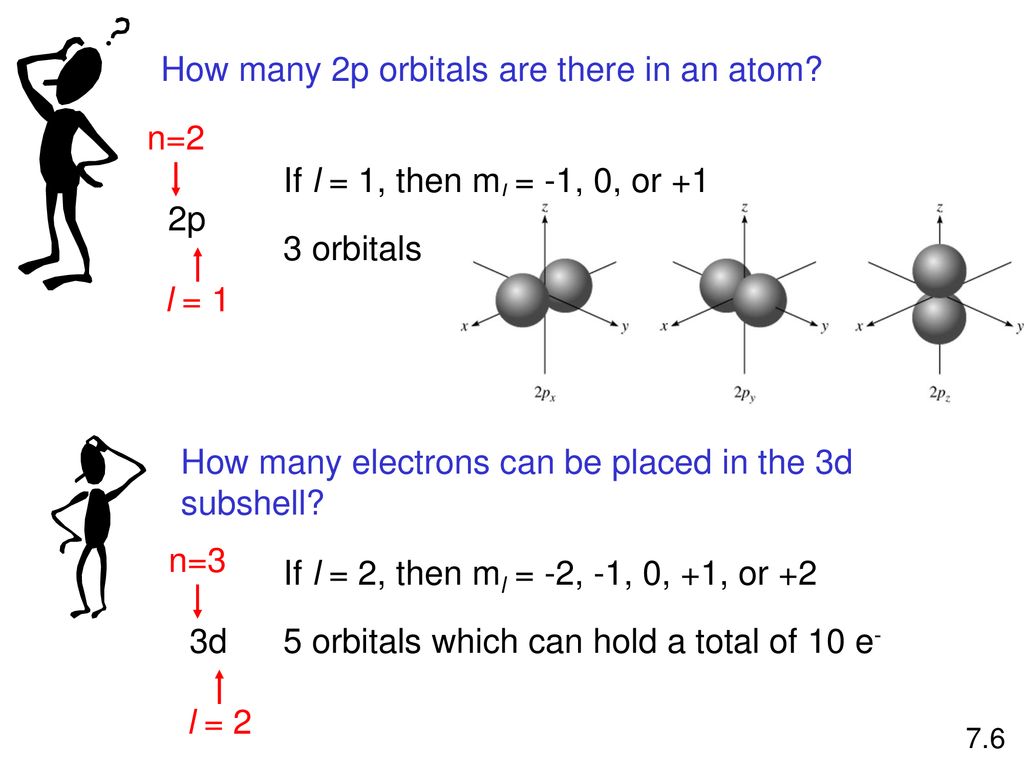
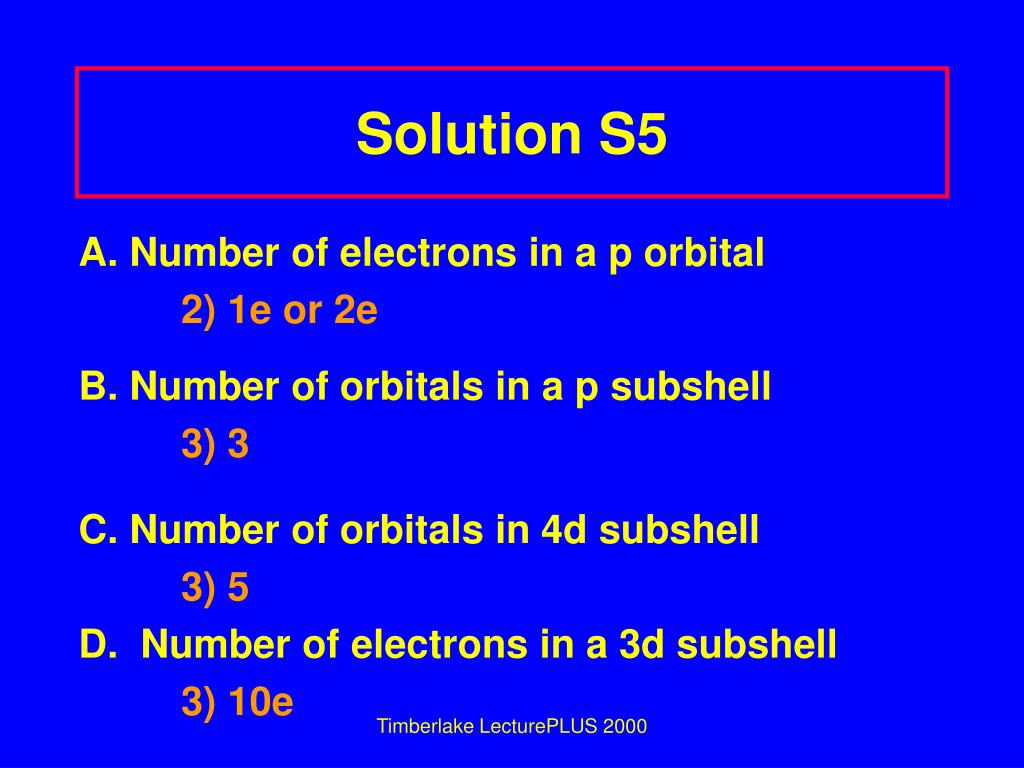
Number 3d electrons how many of those electrons are unpaired. Answer to how many electrons does a co atom have in its 3d subshell. 2nd shell has s and p orbital and can hold upto 8 electrons 3rd shell has s p and d orbital and can hold upto 18 electrons. Number 3d electrons how many of those electrons are unpaire.
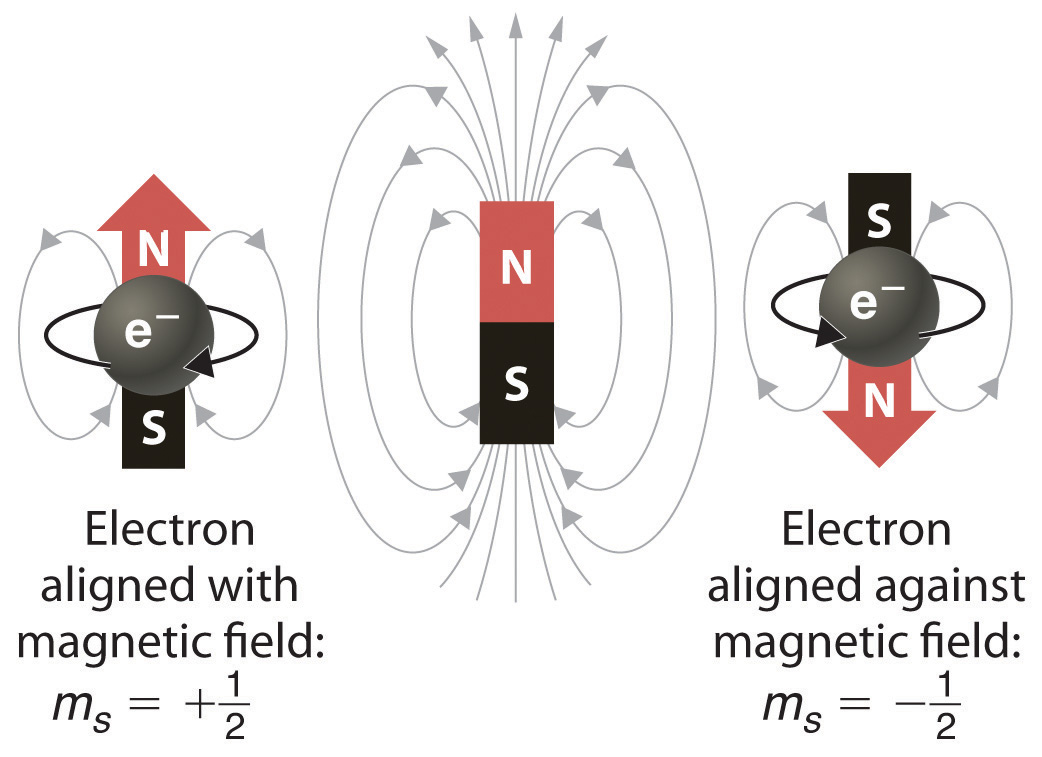
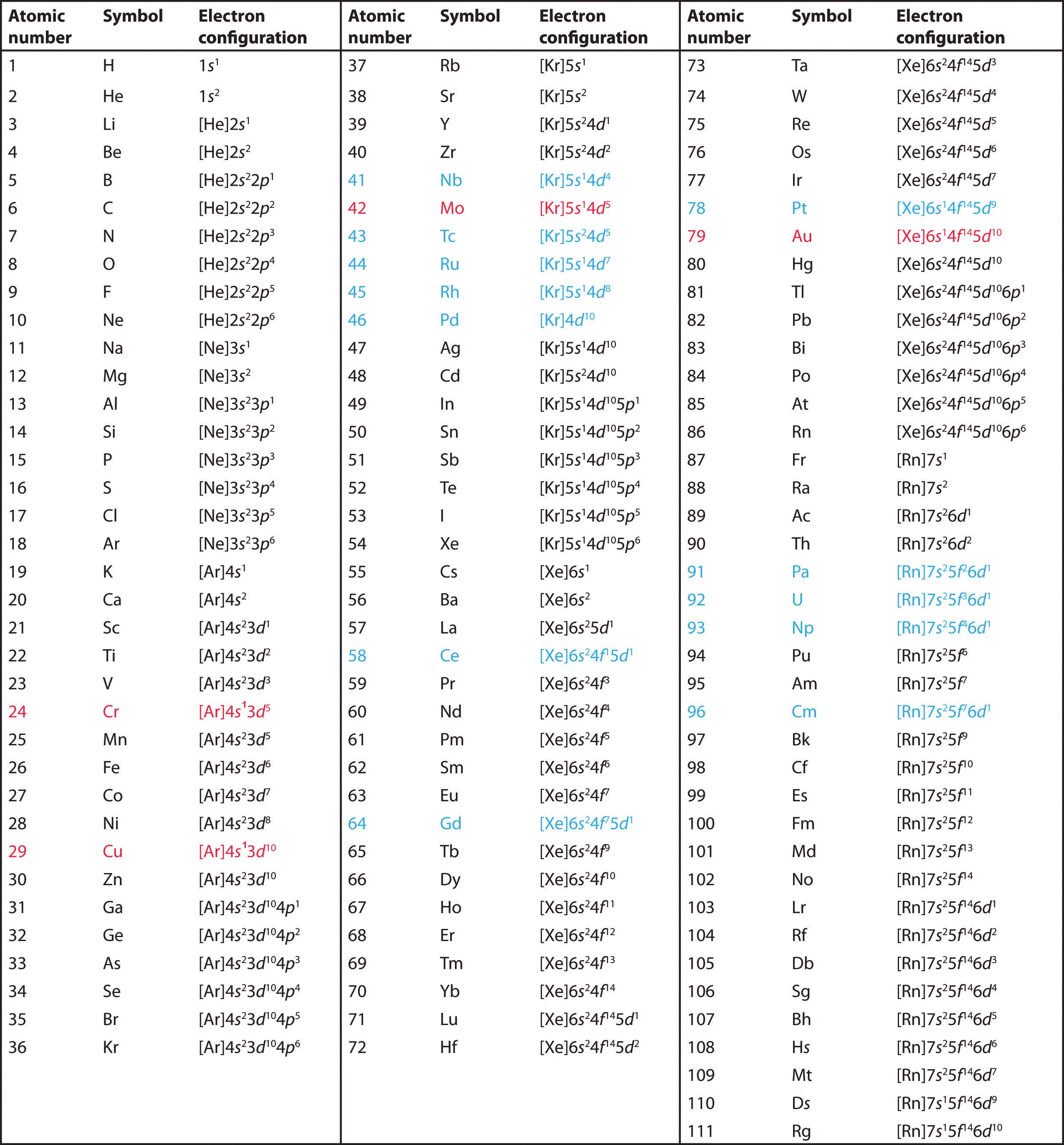
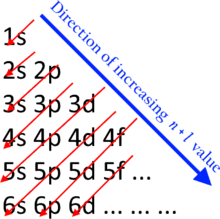
The electron configuration of cobalt is ar3d74s2 a r 3 d 7 4 s 2. 3d electrons 3d electrons how many of those electrons are u. N3 l0 ml0 ms 12.