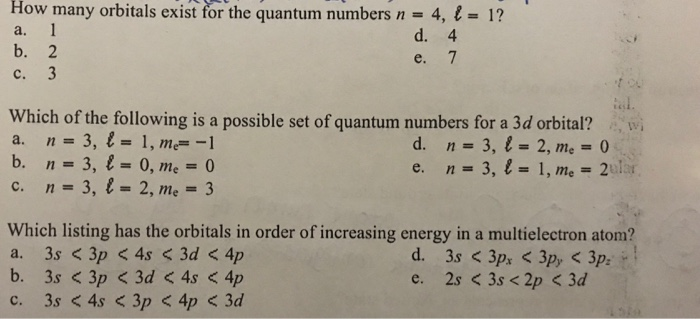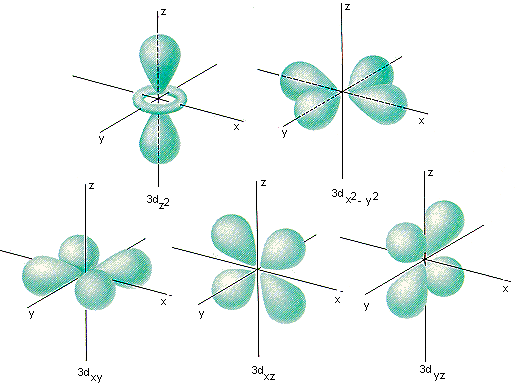How Many Orbitals In 3d
An atomic orbital can hold two electrons.

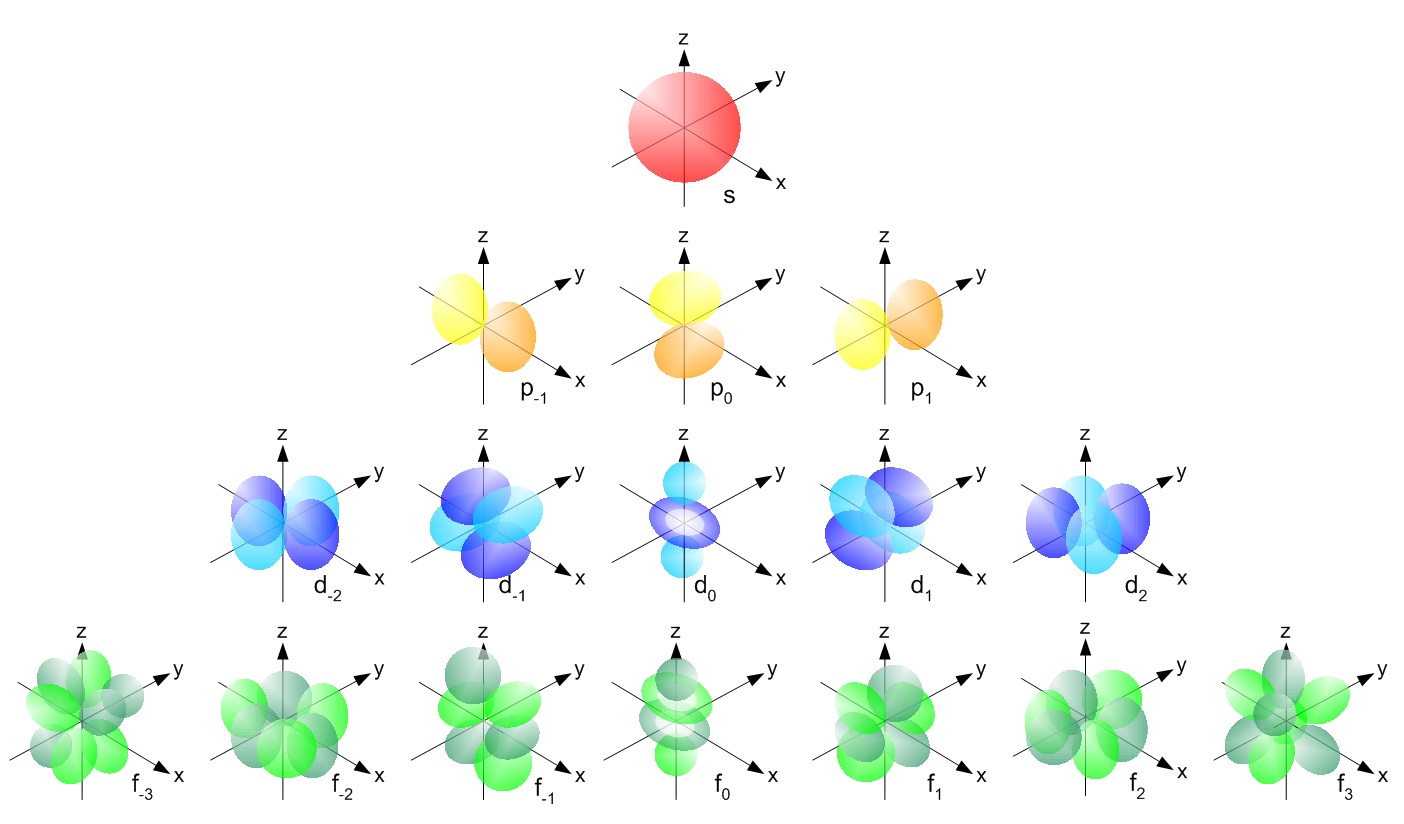
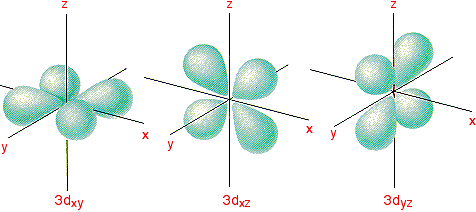
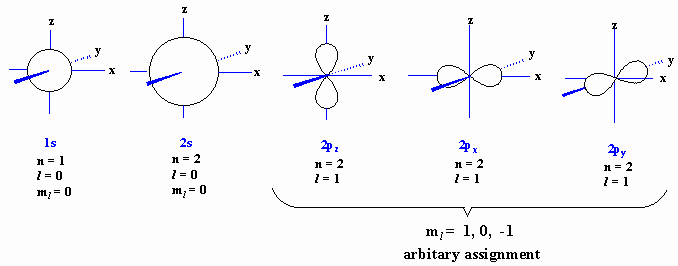
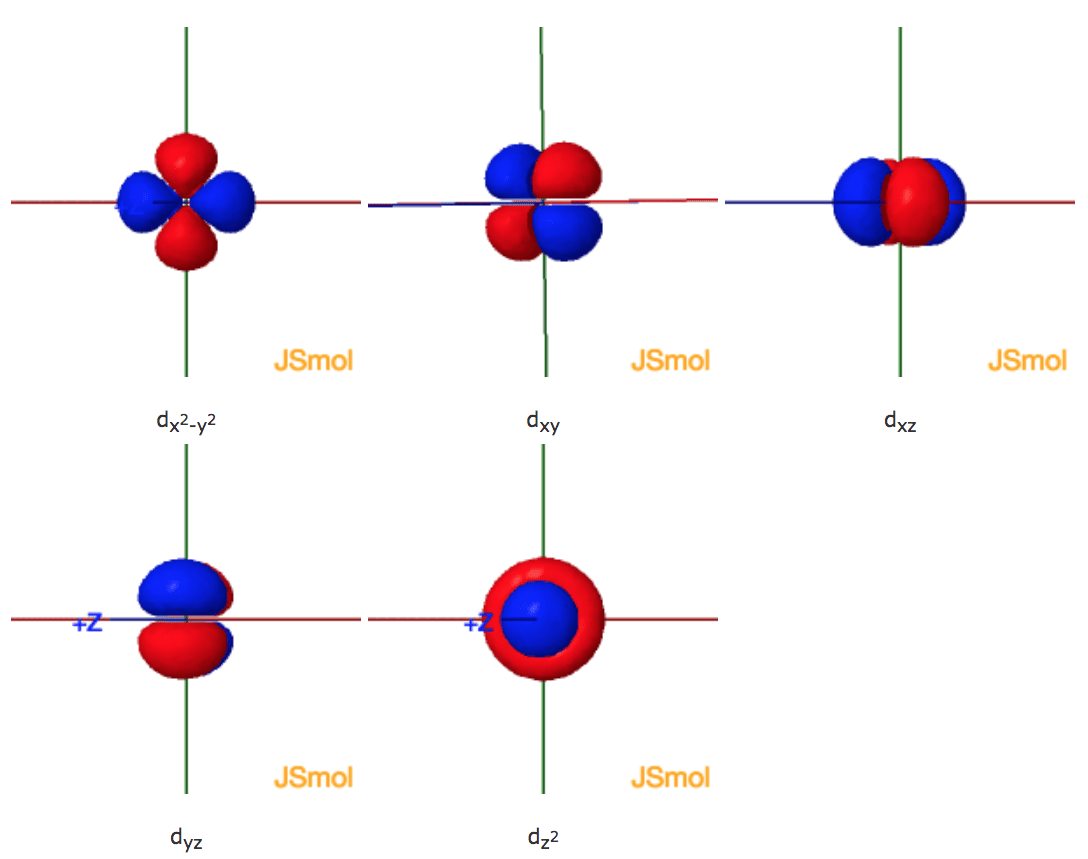
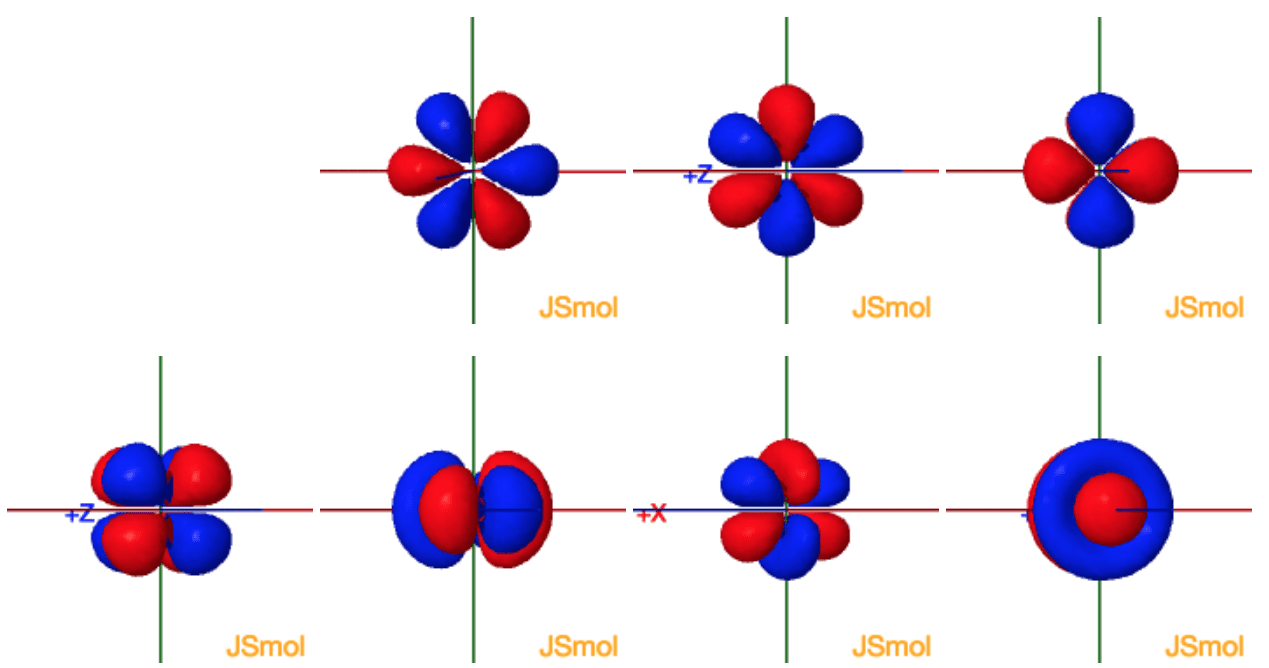
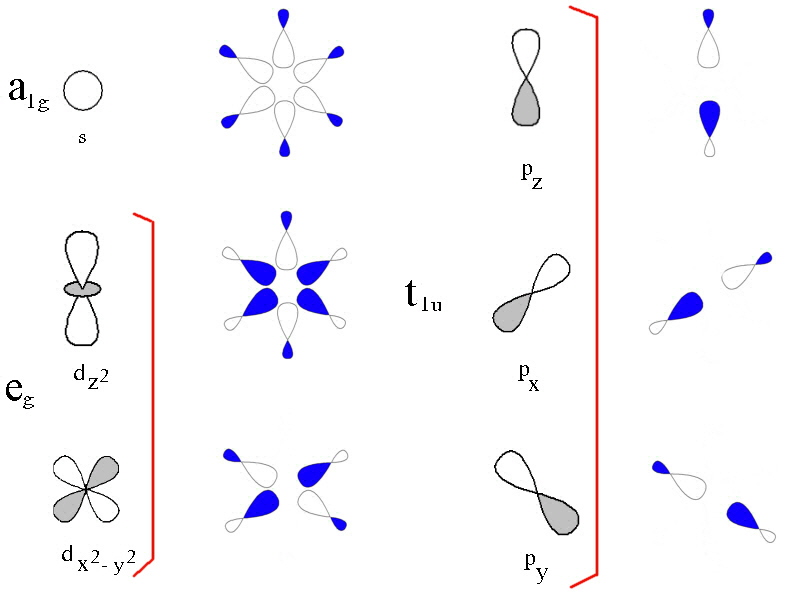
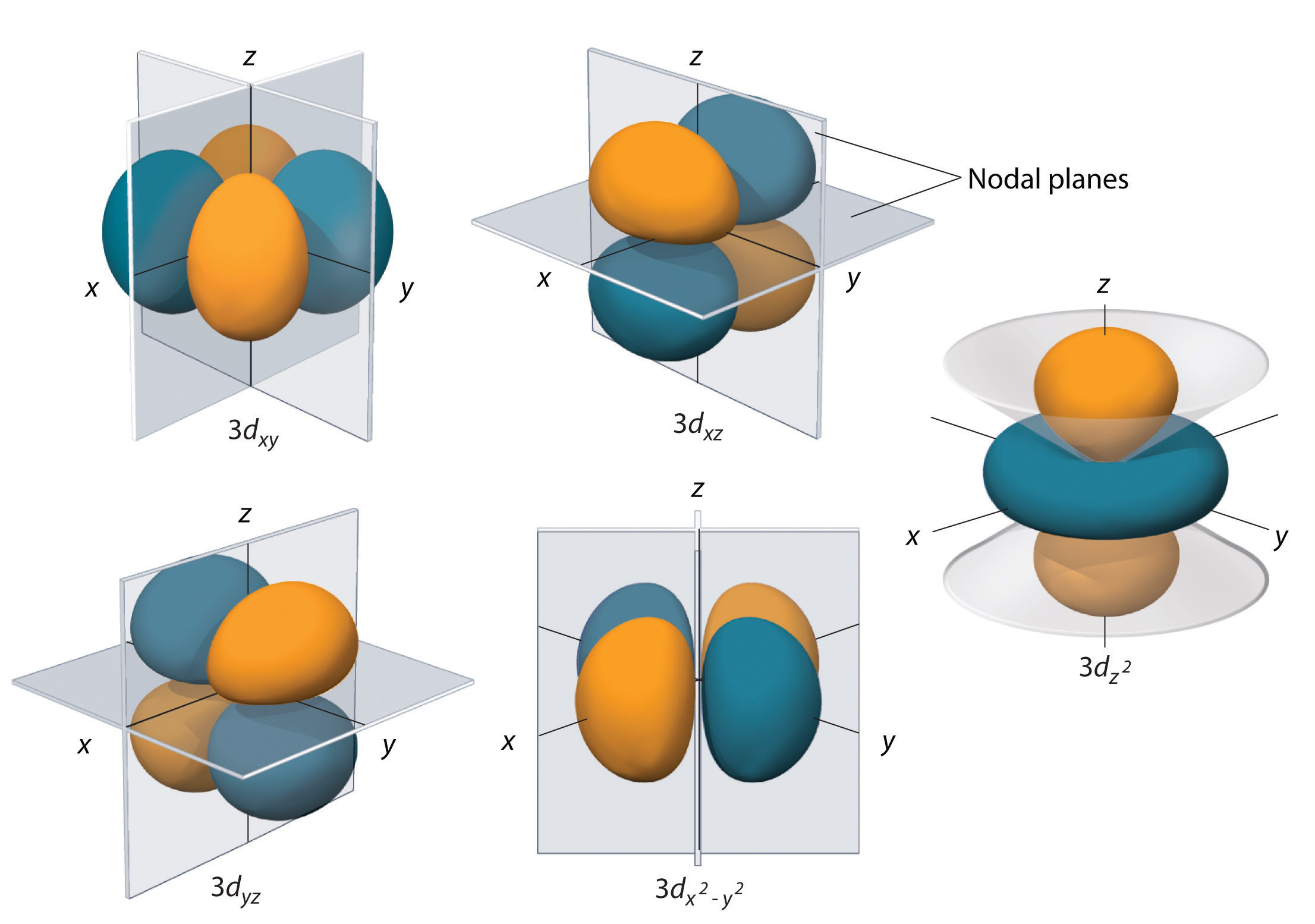
How many orbitals in 3d. The orbital d z 2 is one of the degenerate orbital among the five orbitals of d sub shell represented as d x y d y z d z x d x 2 y 2 d z 2. Four of the five 3d orbitals consist of four lobes arranged in a plane that is intersected by two perpendicular nodal planes. In 3p there are 3 orbitals. In 3 d z 2 3 represents the principal quantum number n.
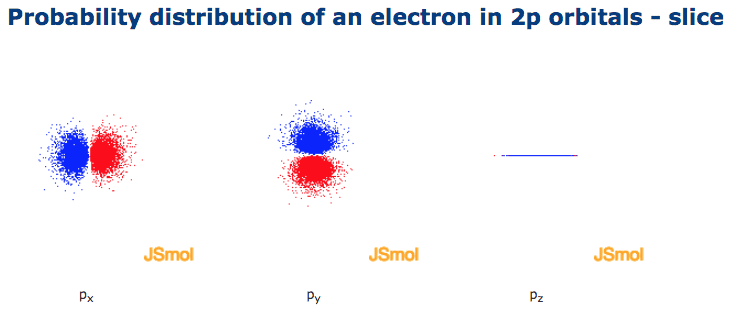
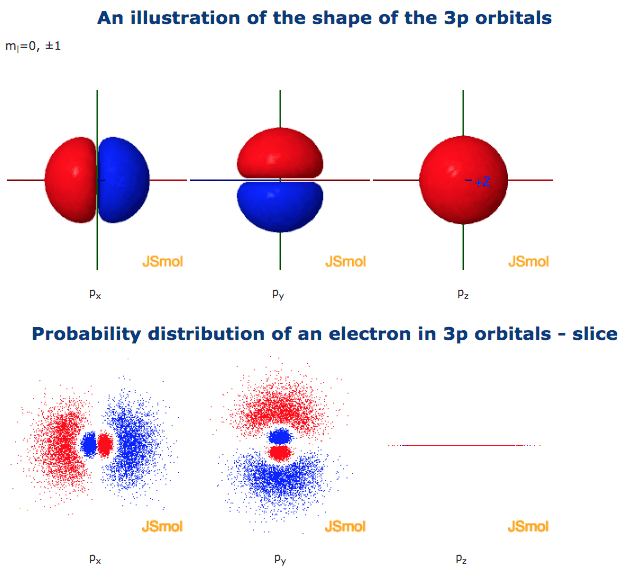
The three orbitals of 5 p are represented as 5 p x 5 p y 5 p z. The p sublevel has 3 orbitals so can contain 6 electrons max. 2 therefore the 3d subshell will contain a total of five 3d orbitals. These four orbitals have the same shape but different orientations.
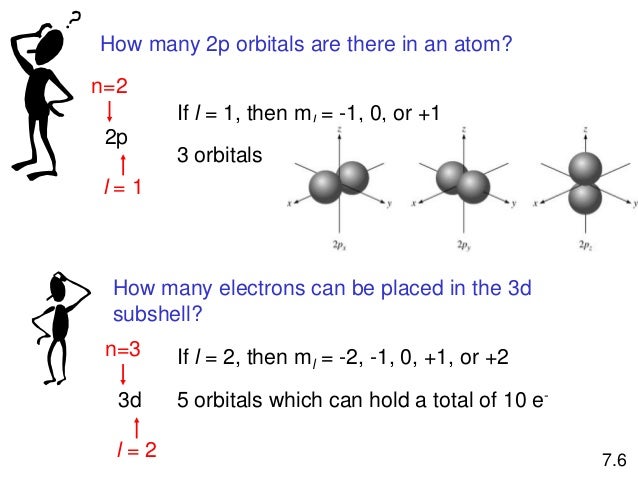
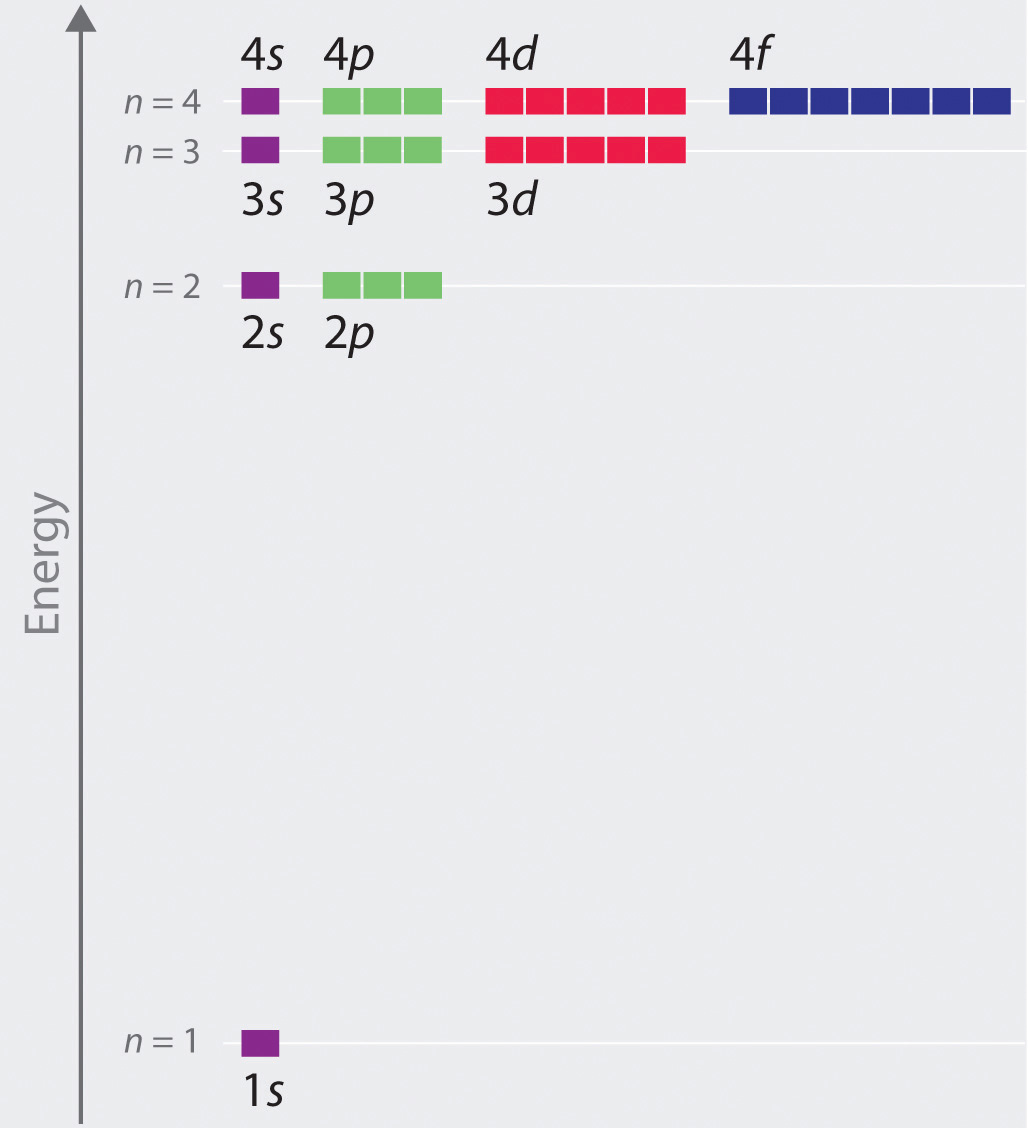
In 3d there are 5 orbitals. Total number of orbitals 9. The reduction in repulsion more than compensates for the energy needed to do this. The fifth 3d orbital 3dz2 has a distinct shape even though it is mathematically equivalent to the others.
If we add them up we get an overall of 9 orbitals. For the number of electrons that can be present in the 3rd shell we use the 2n2 formula. Introducing a second electron into a 3d orbital produces more repulsion than if the next electron went into the 4s orbital. How many orbitals in an atom can have the designation eq5p 3dz2 4d n 5 n 4 eq.
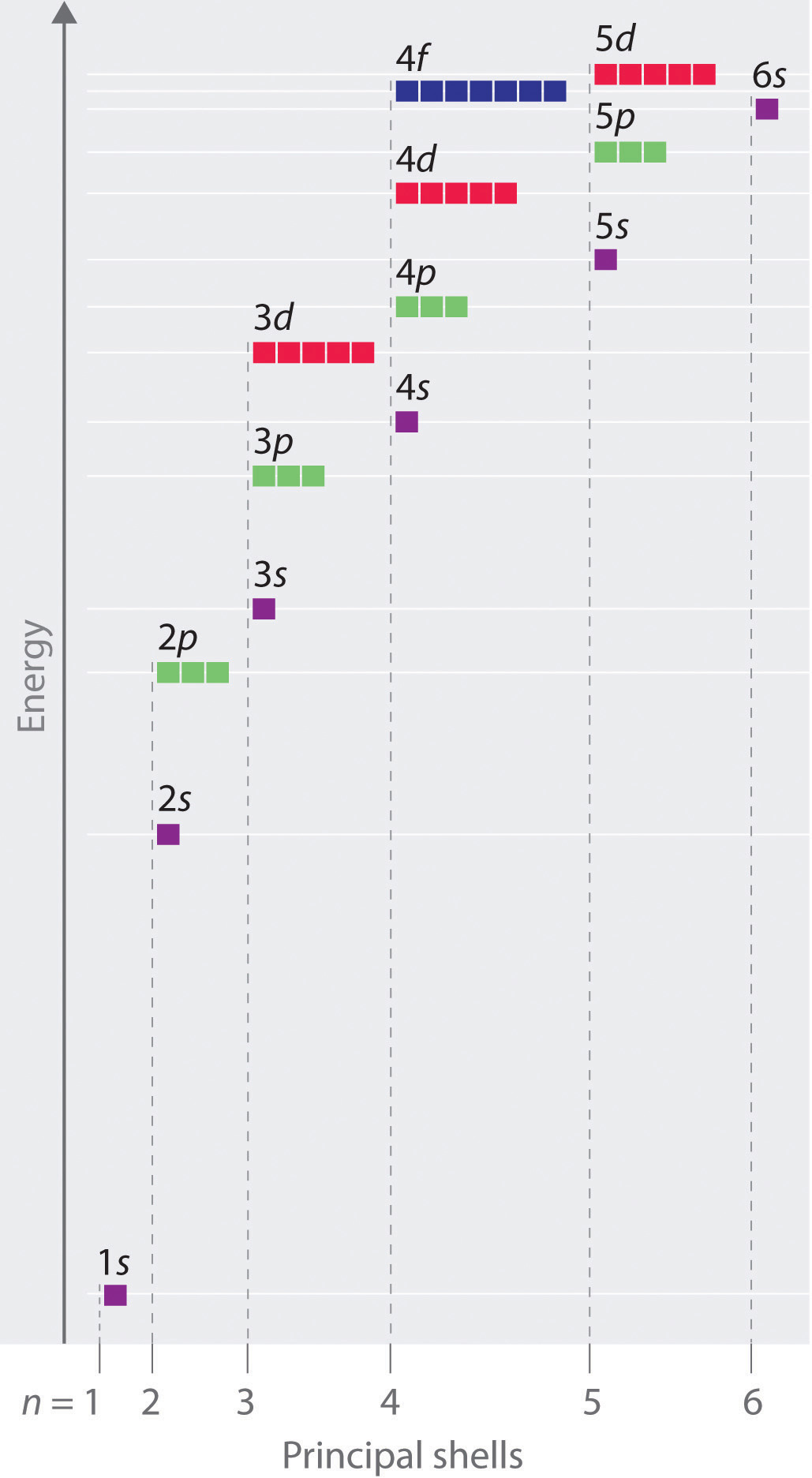
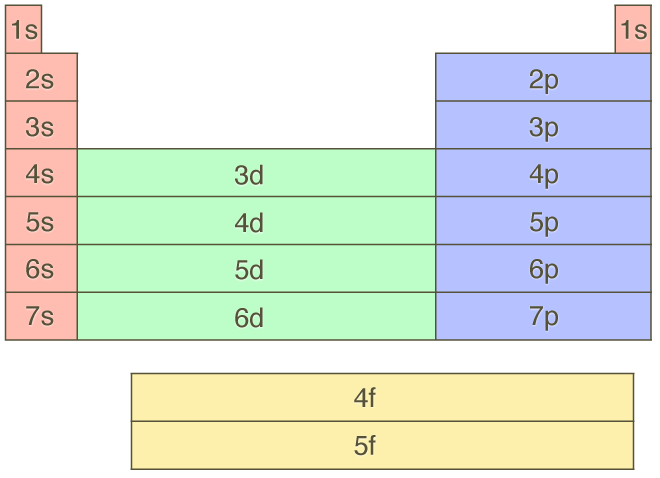
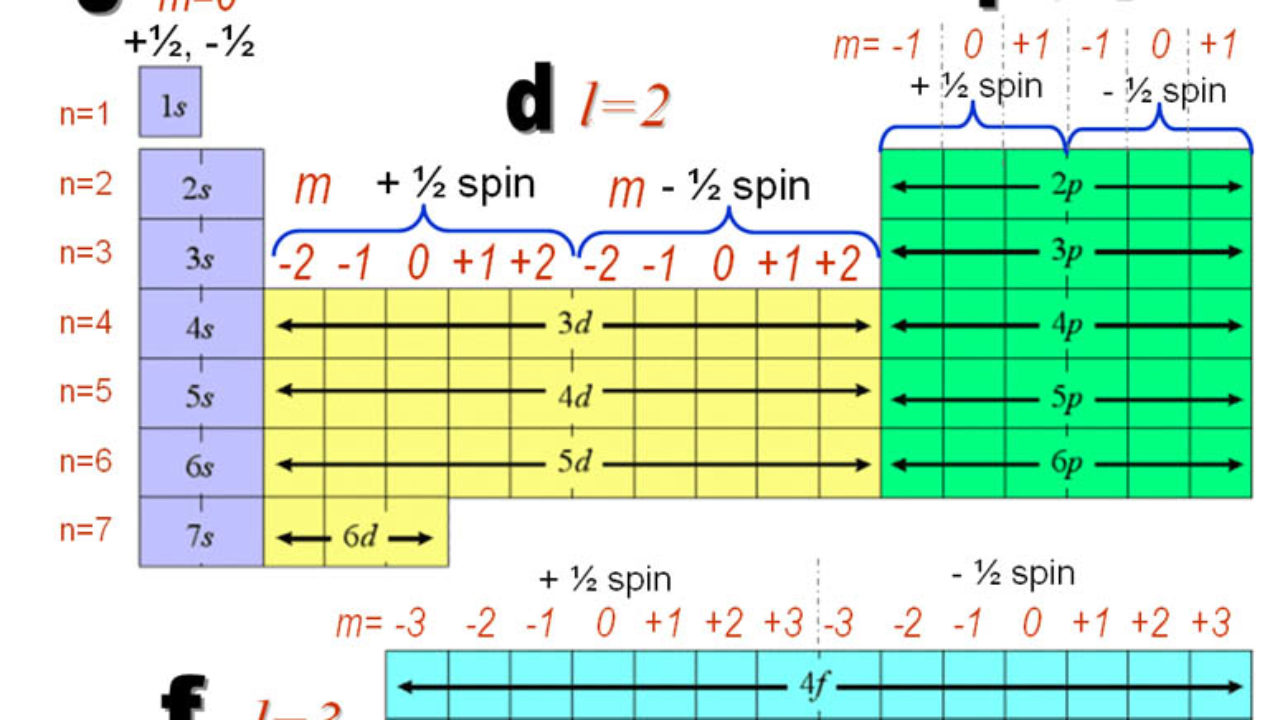
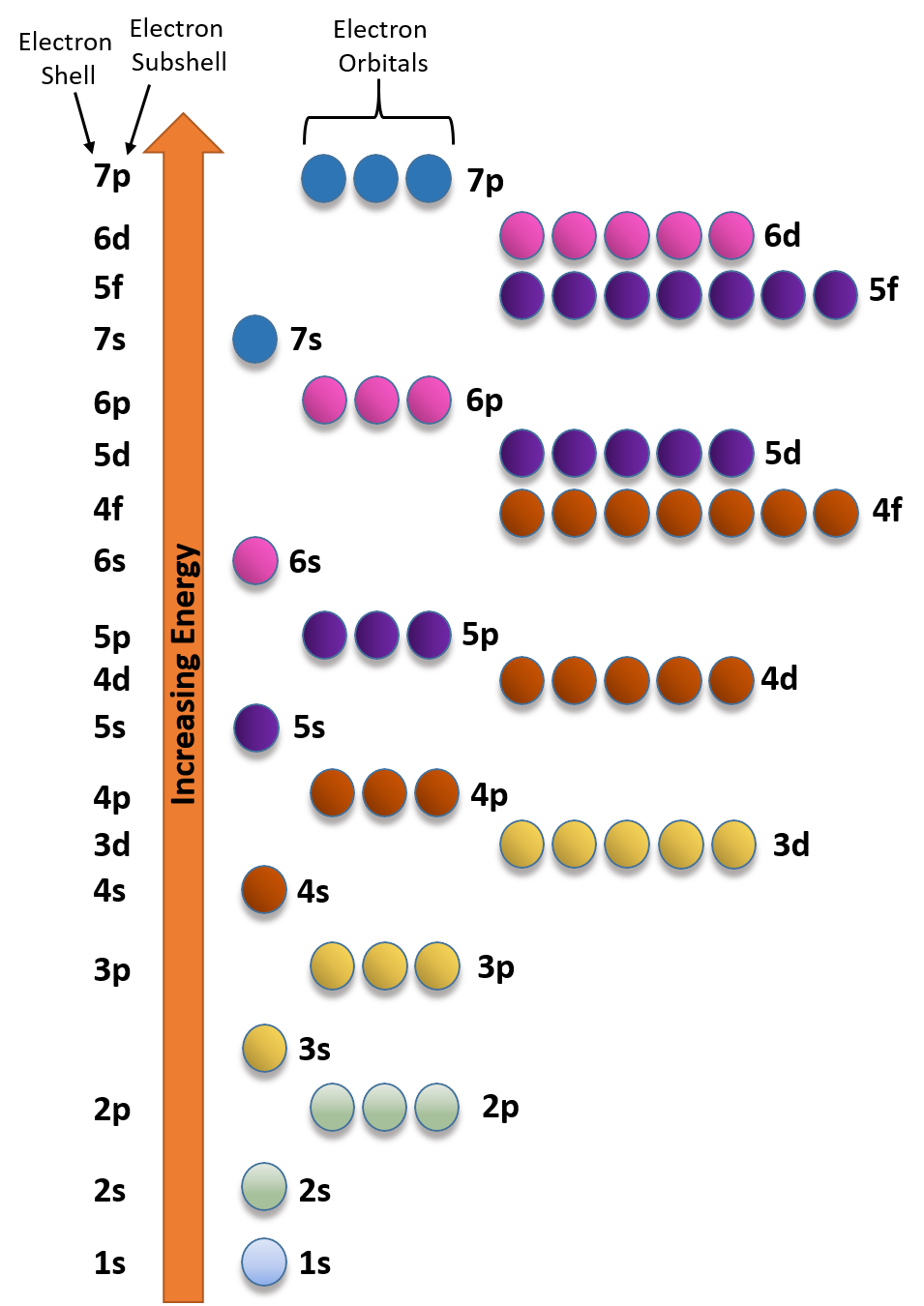
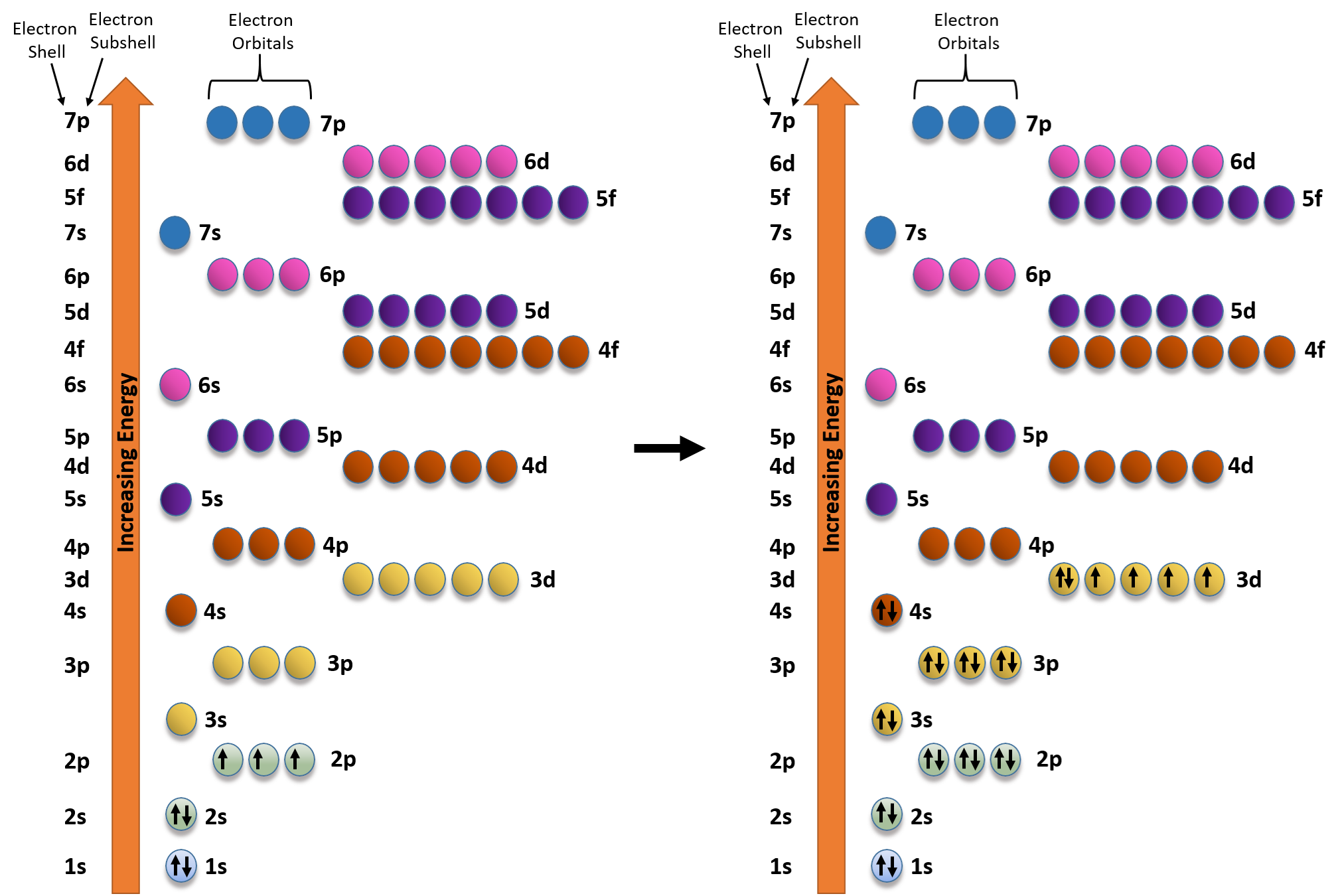
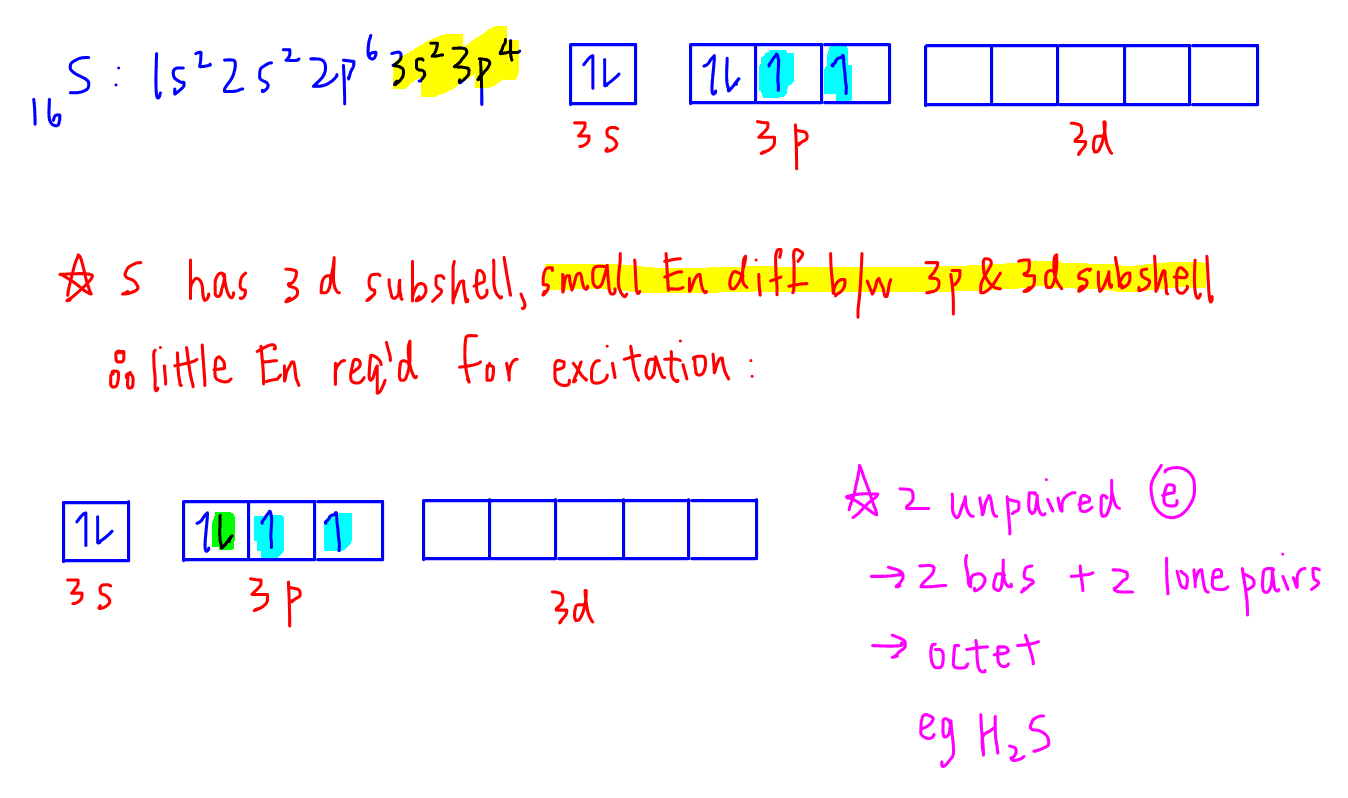
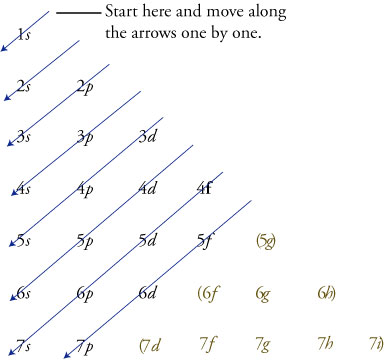
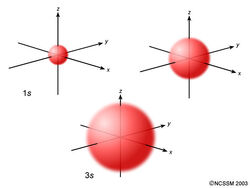
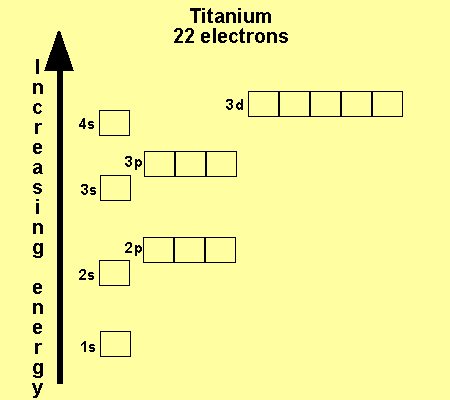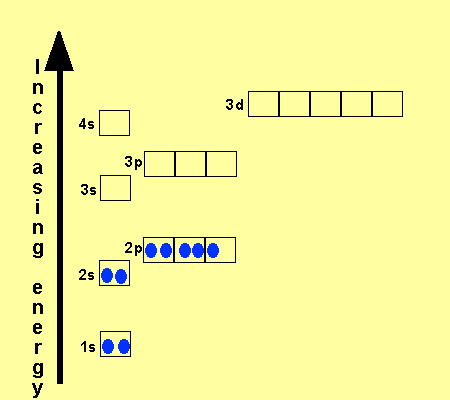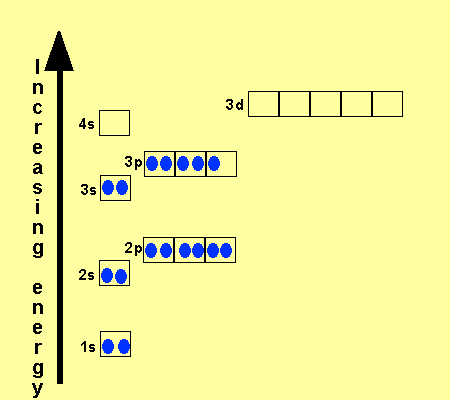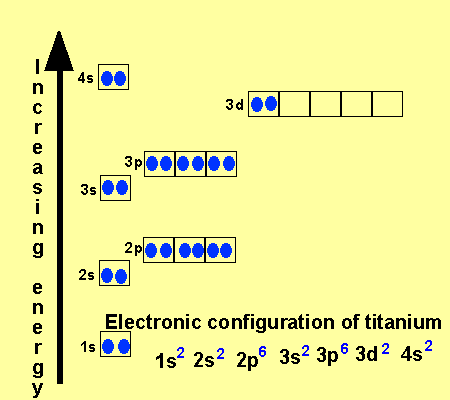
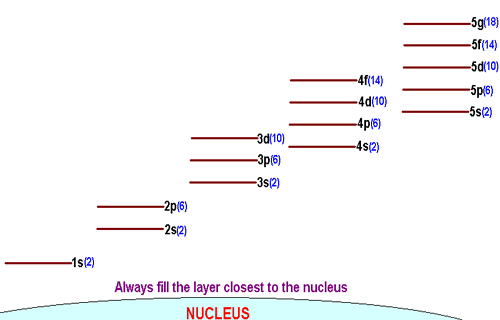
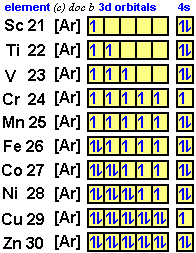
In the picture below the orbitals are represented by the boxes. The distribution of electrons among the orbitals of an atom is called the electron configurationthe electrons are filled in according to a scheme known as the aufbau principle building up which corresponds for the most part to increasing energy of the subshells. Likewise the 4d subshell will contain a total of five 4d orbitals the 5d subshell will contain a total of five 5d orbitals and so on. In the 3s there is one orbital.
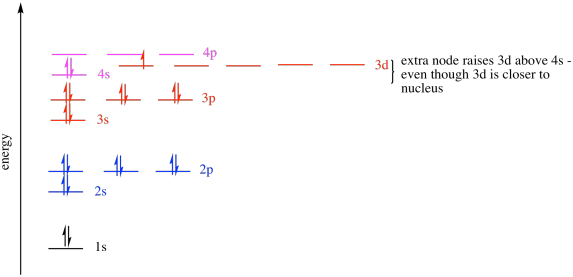
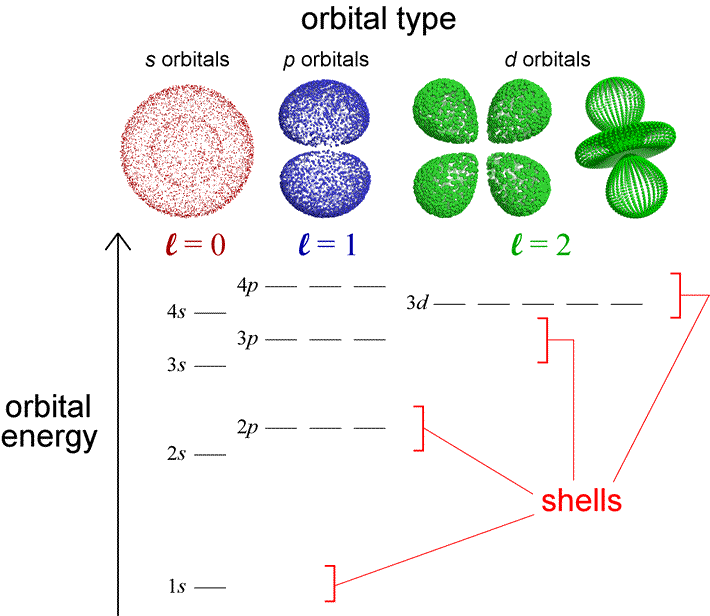
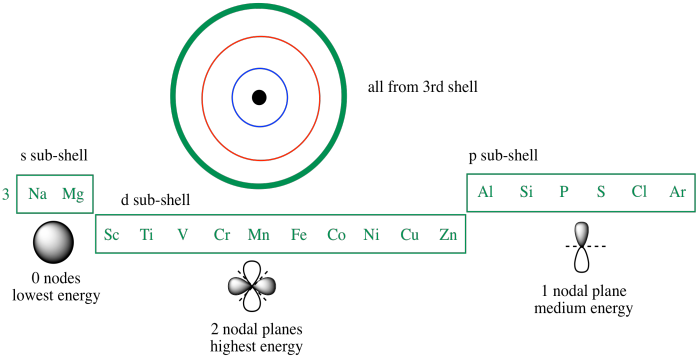
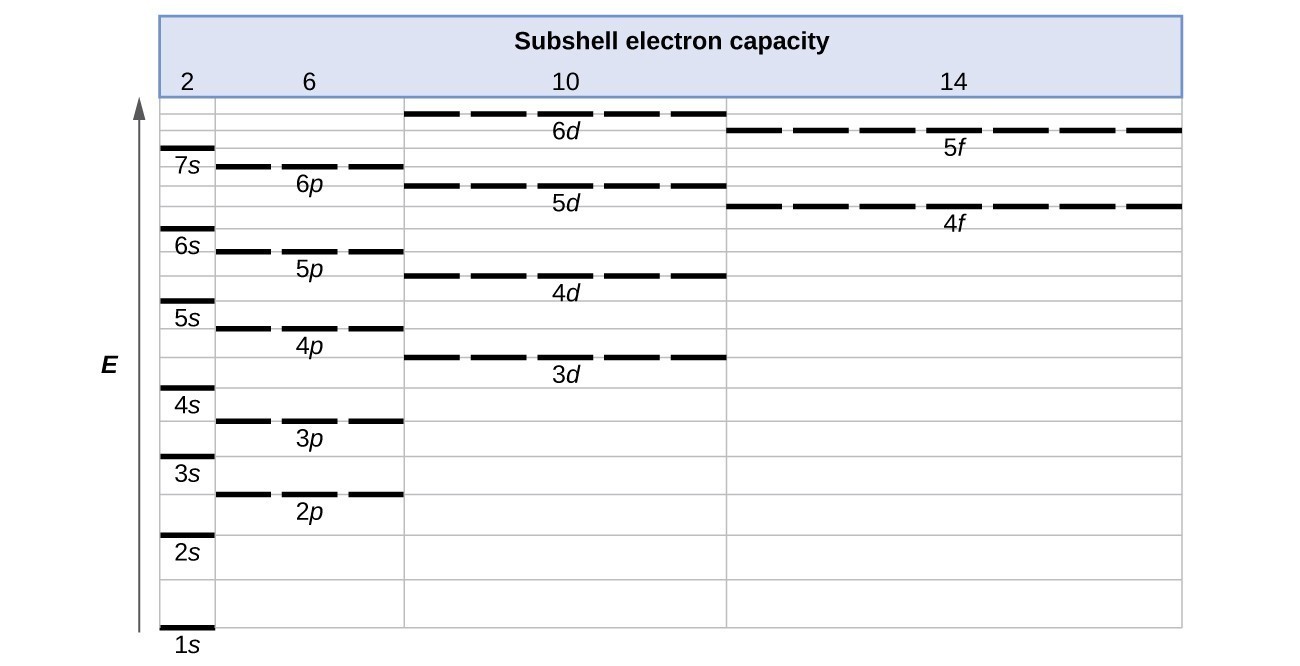
The 3d orbitals are quite compactly arranged around the nucleus. Each d orbital has 5 sub orbitals. The s sub shell has 1 orbital while p sub shell contains 3 orbitals and d sub shell has 5 orbitals. There isnt a very big gap between the energies of the 3d and 4s orbitals.
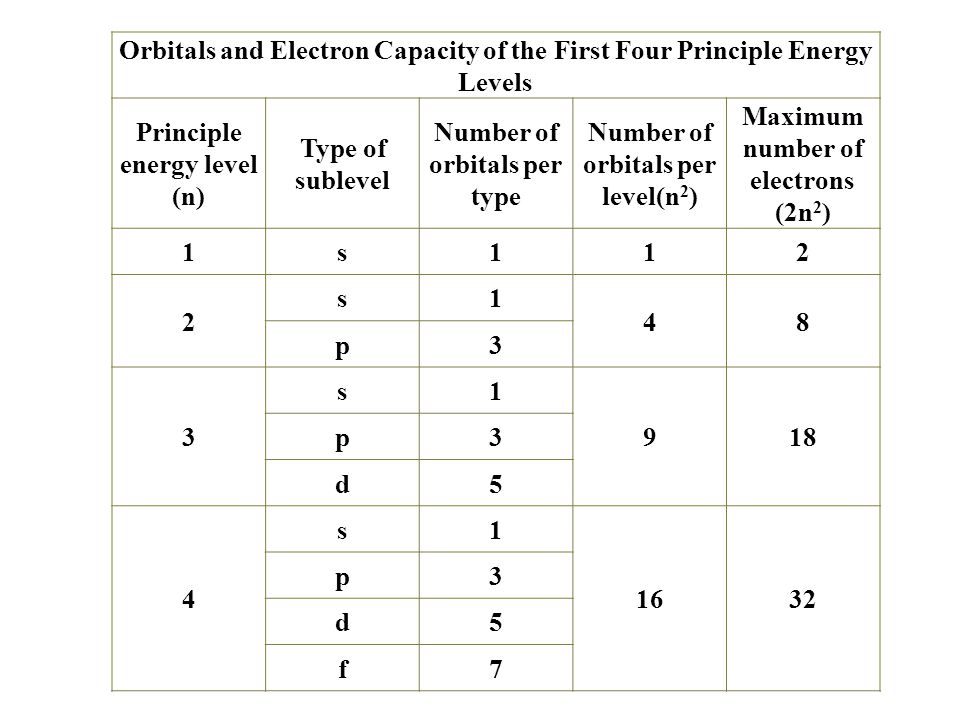
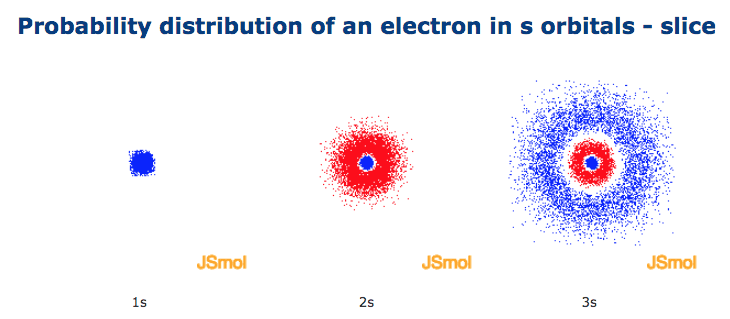
Hence 3 d z 2 in an atom has one orbital. And the 4 sublevel has 7 orbitals so can contain 14 electrons max. The d sublevel has 5 orbitals so can contain 10 electrons max. The 3rd shell where n3 has the orbitals 3s 3p 3d.
This mens that any d subshell youll ever run into will have a total of five d orbitals described by ml 2. You can put two electrons in each box.