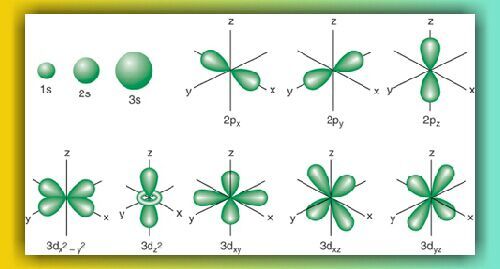
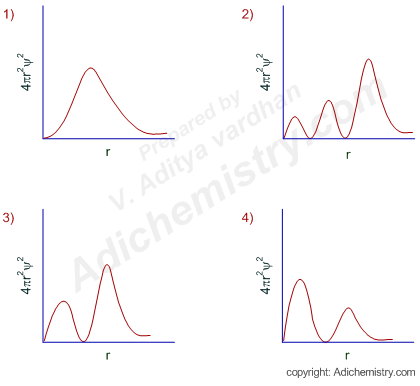
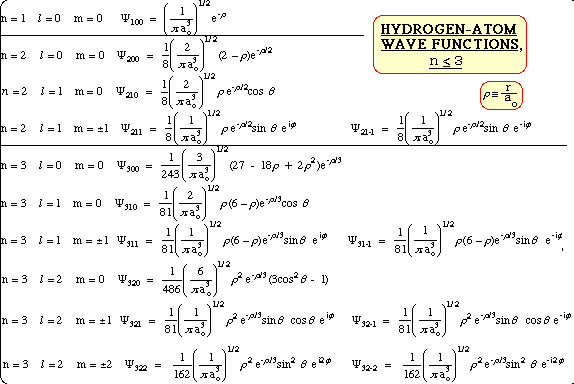
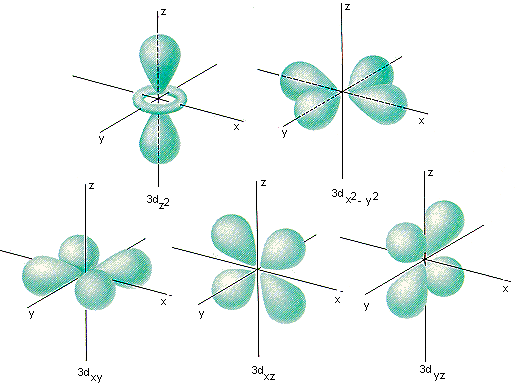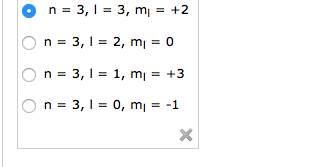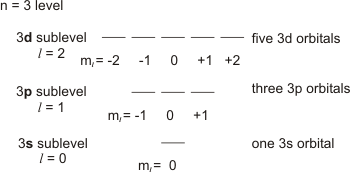3d Orbital Quantum Numbers
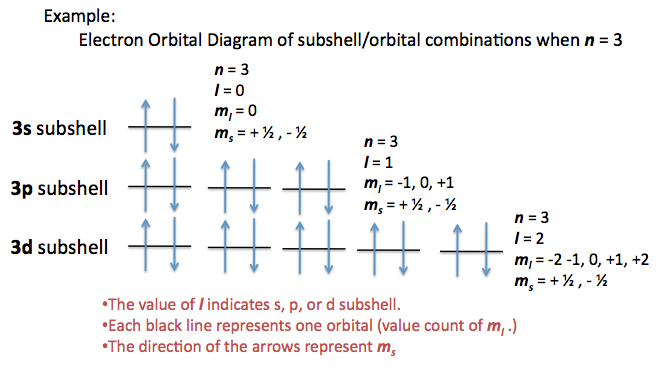
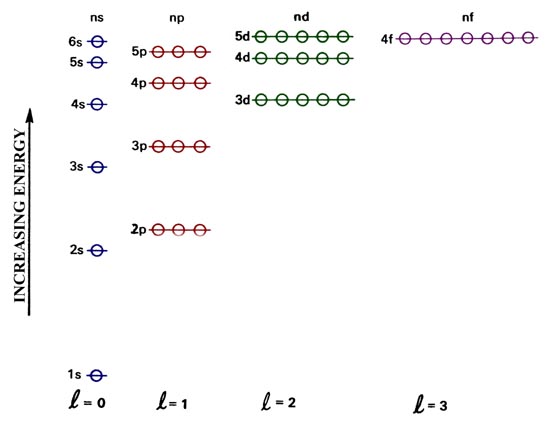
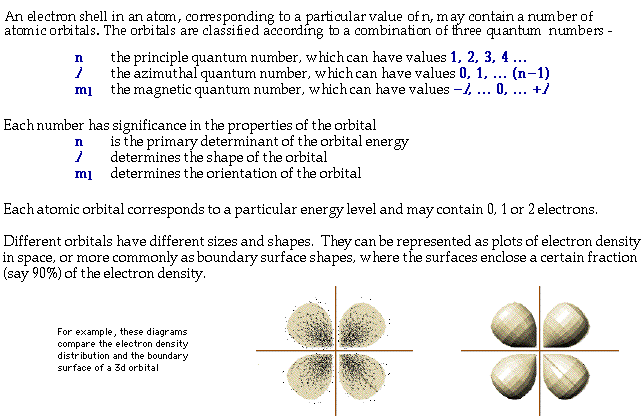
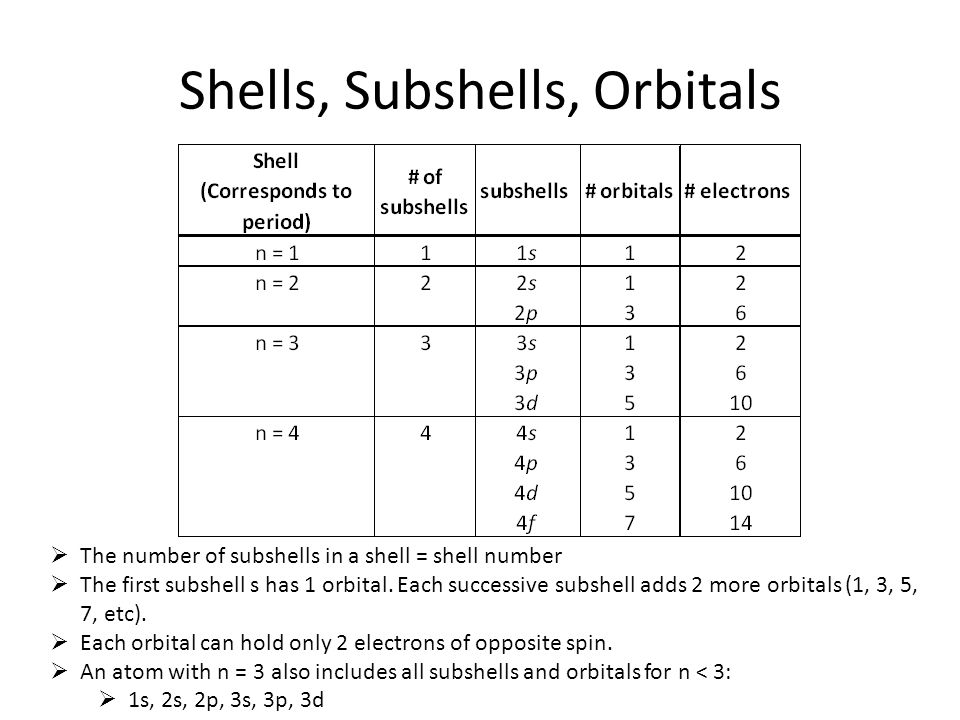
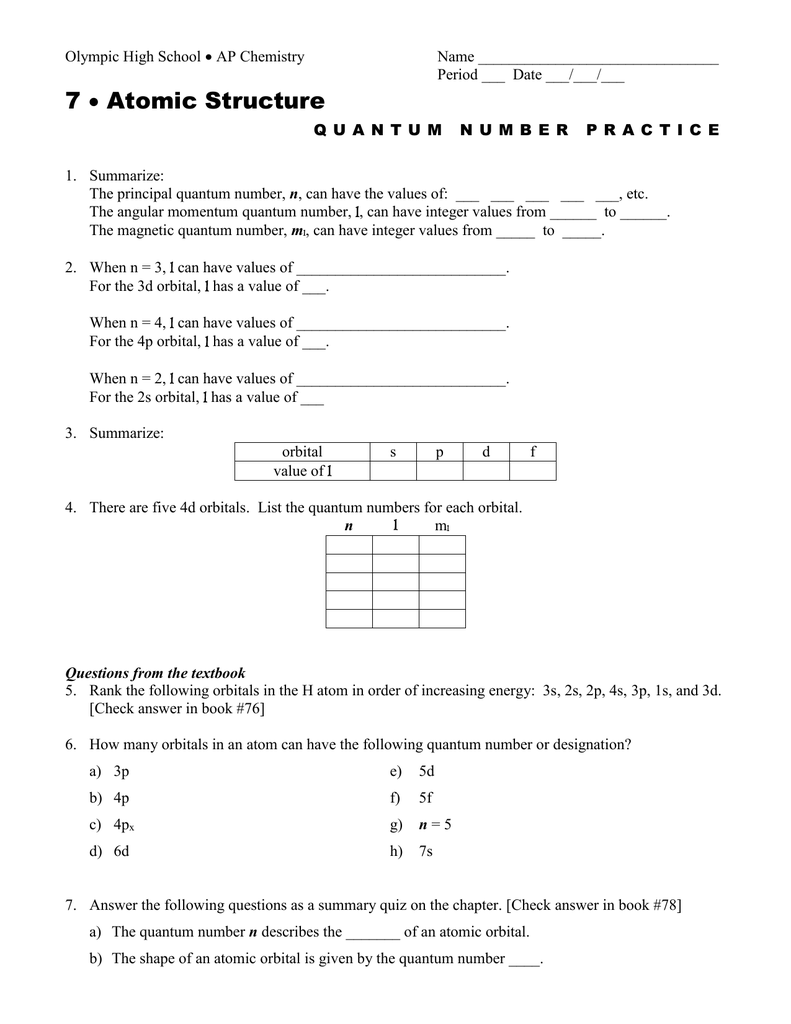
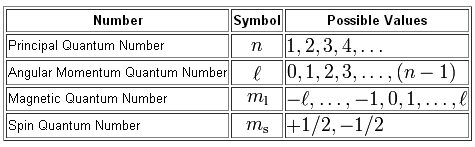
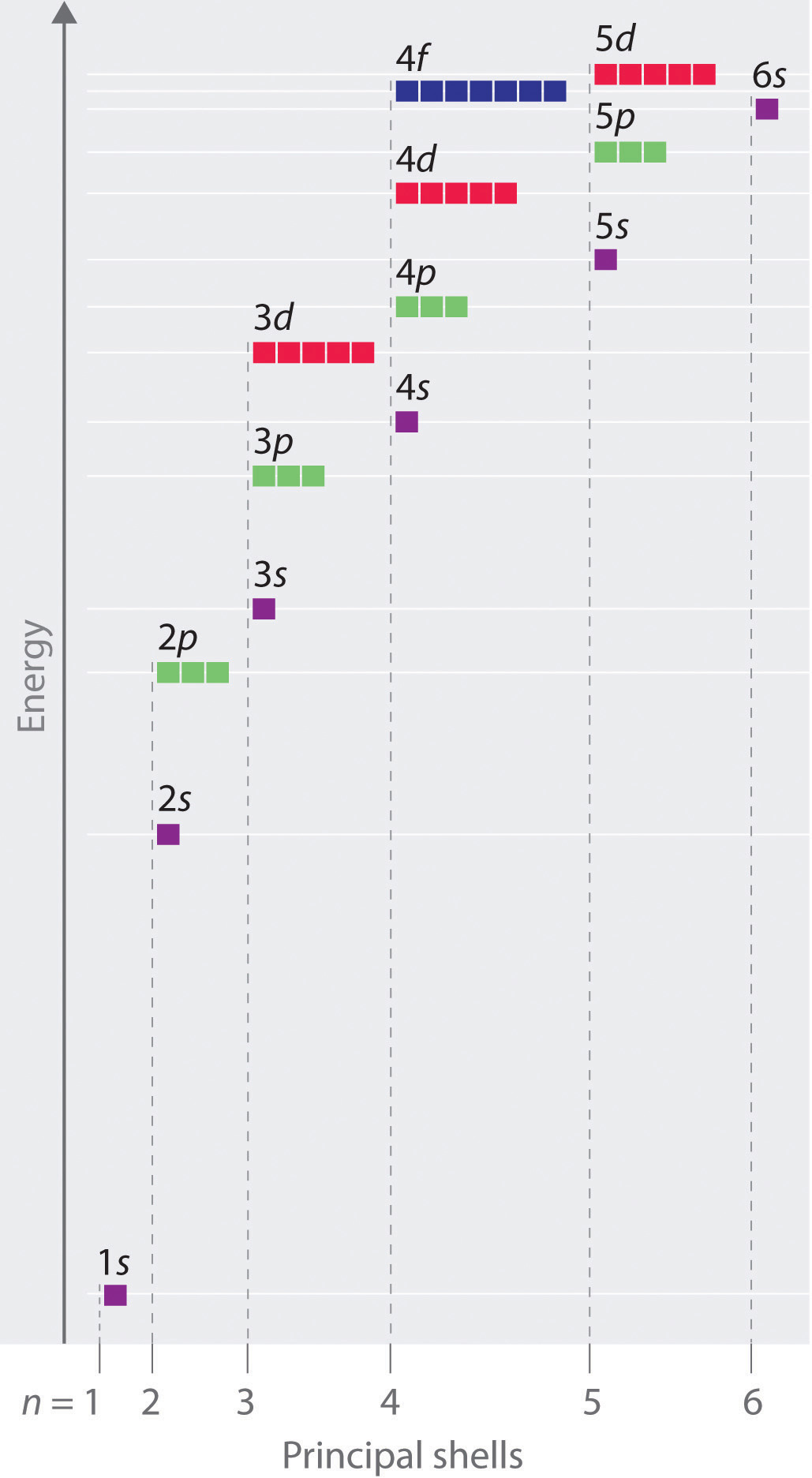
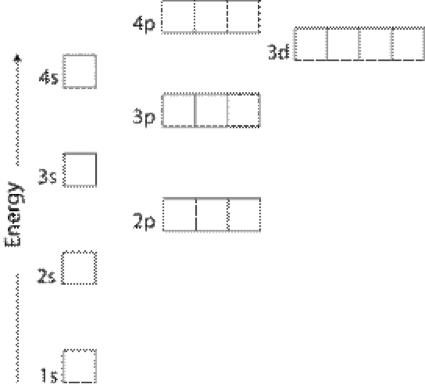
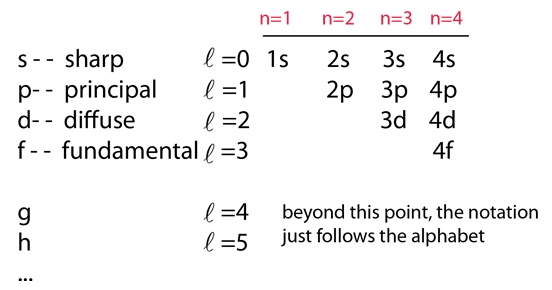
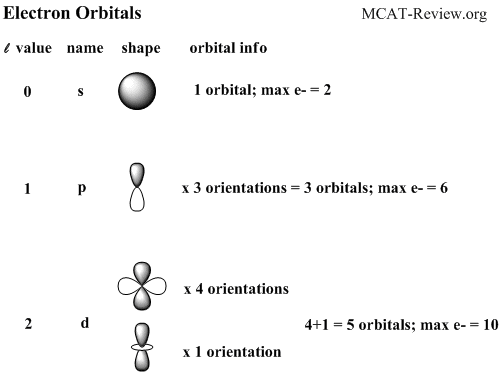
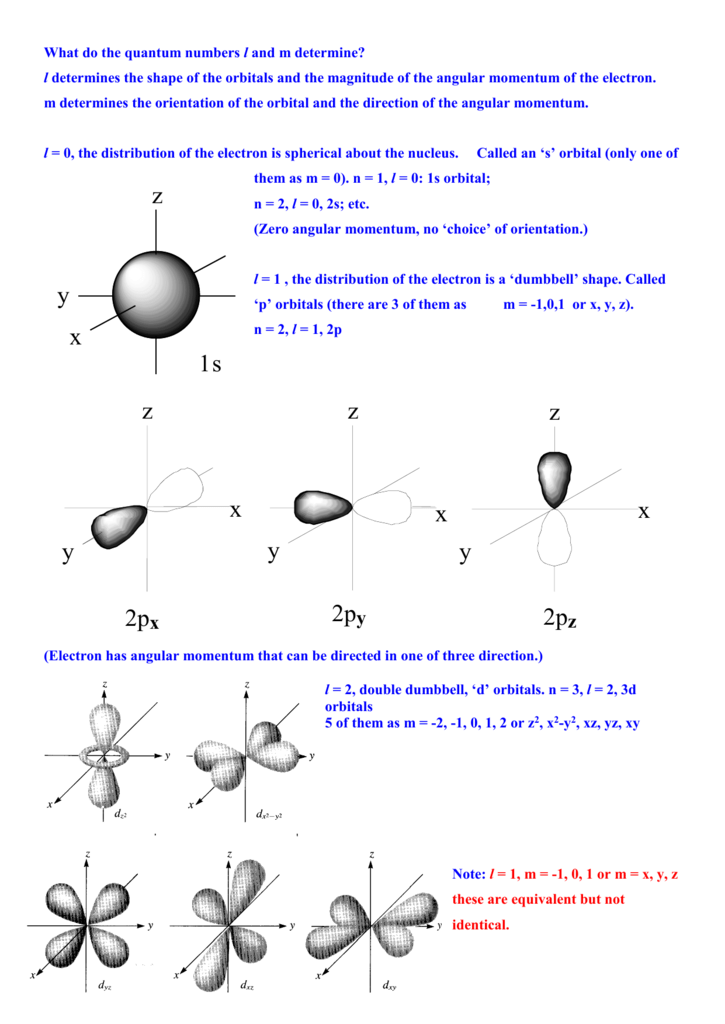
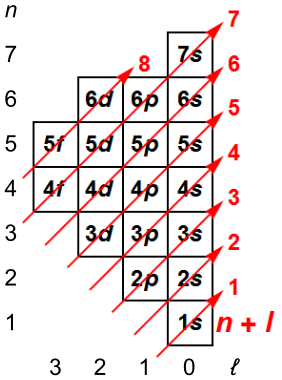
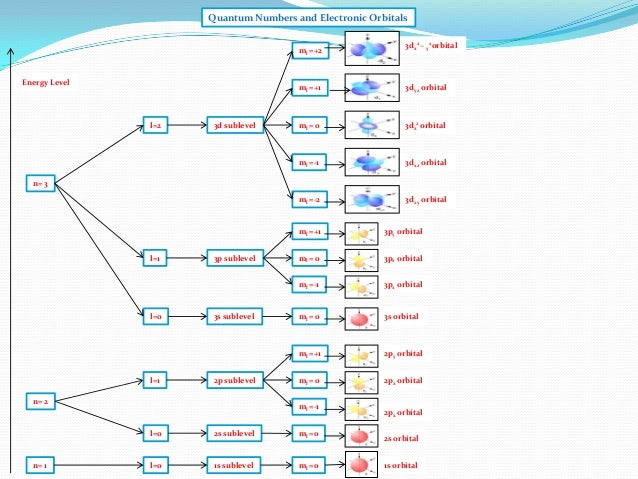
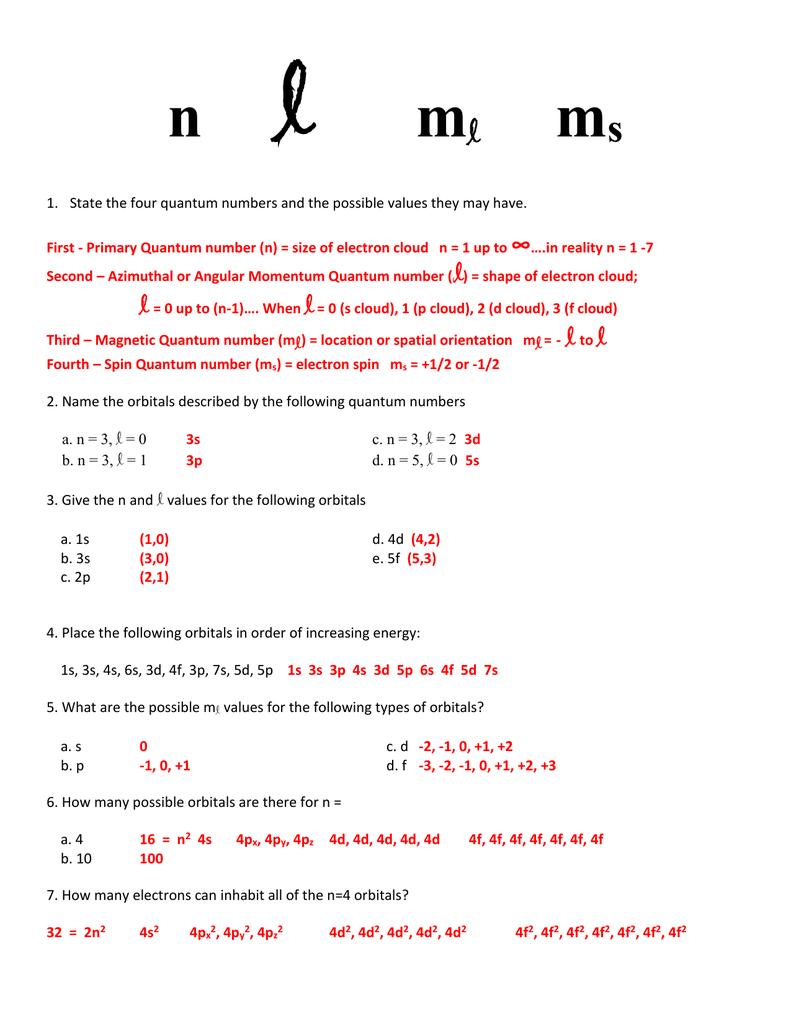
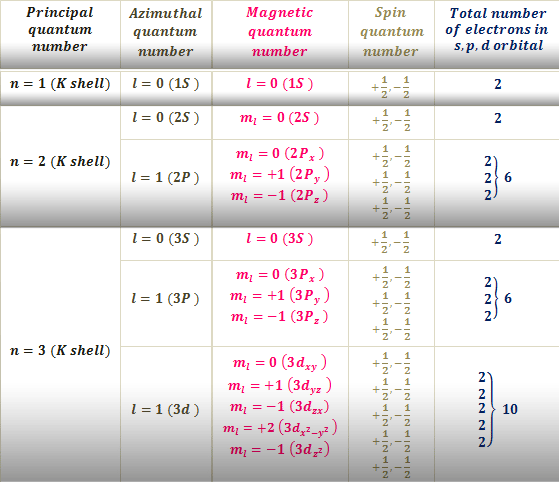
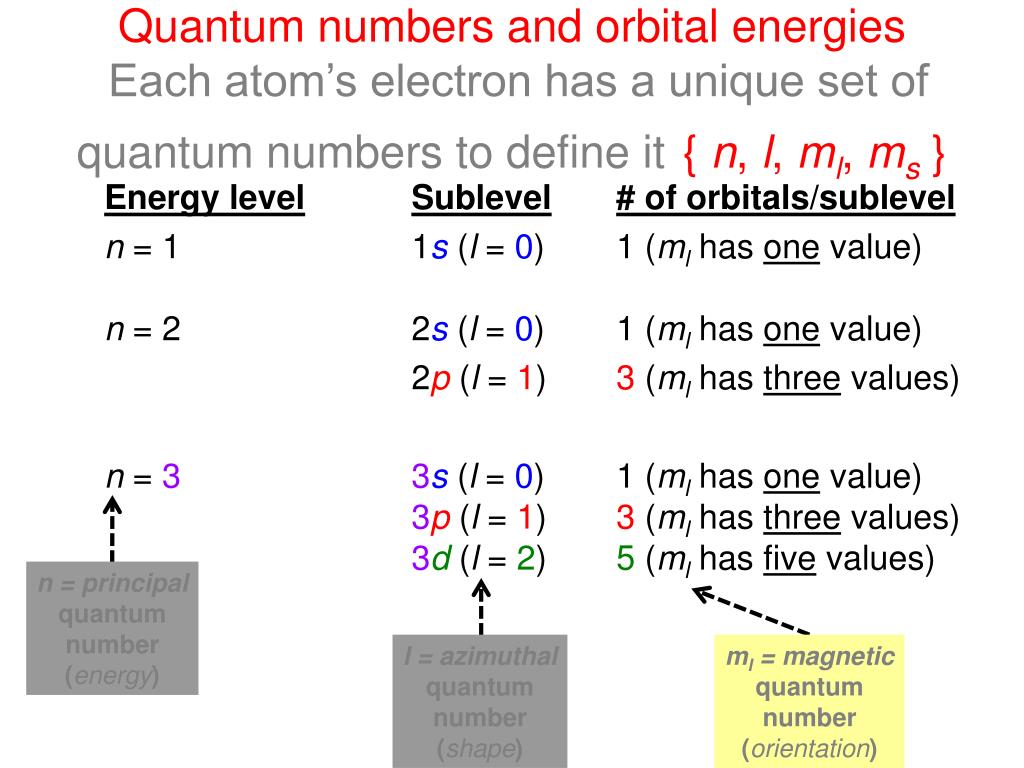
Principal quantum number n energy level in orbitals and its value could be any positive integer starting from 1 angular momentum quantum number l has to be at least 1 less than n range of values from 0 up to n 1 and each number corresponds to a subshell.
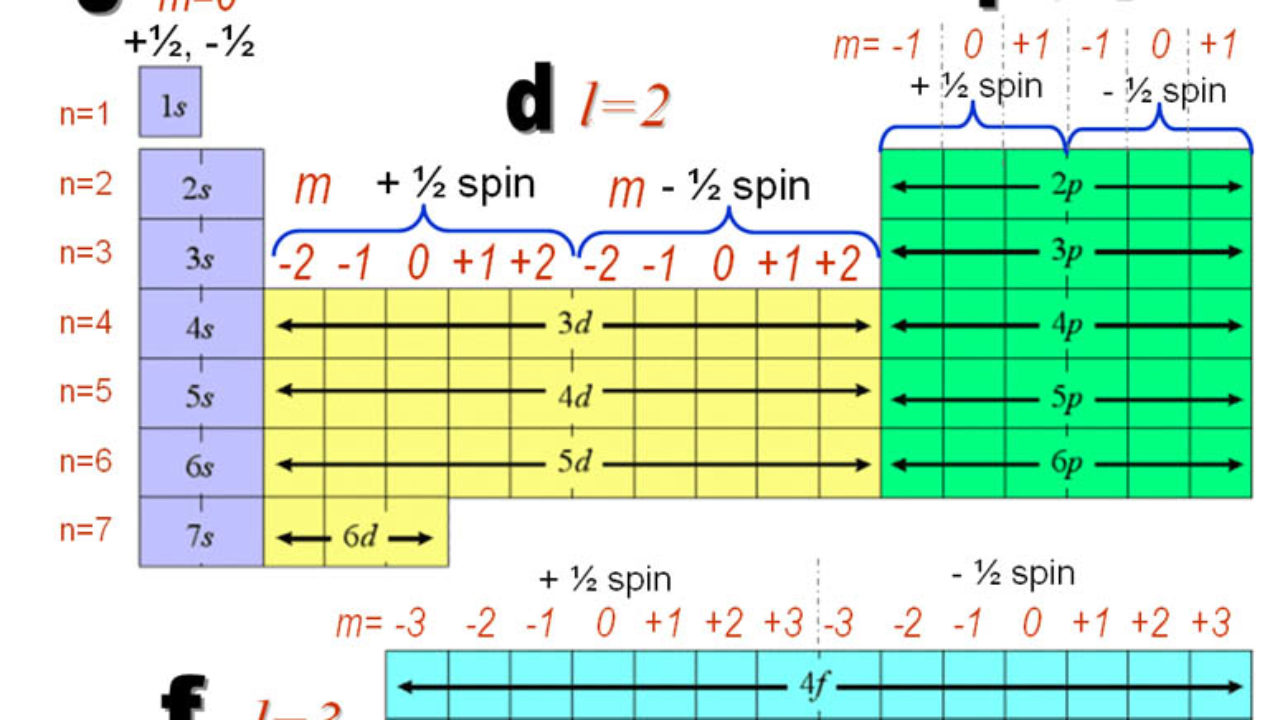
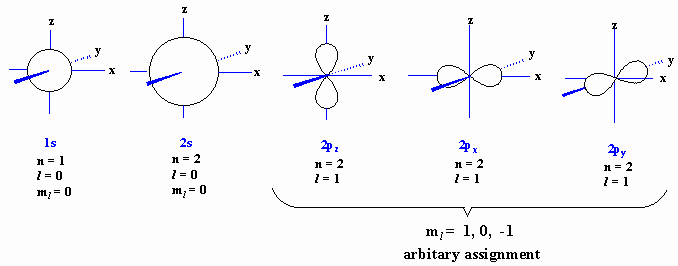
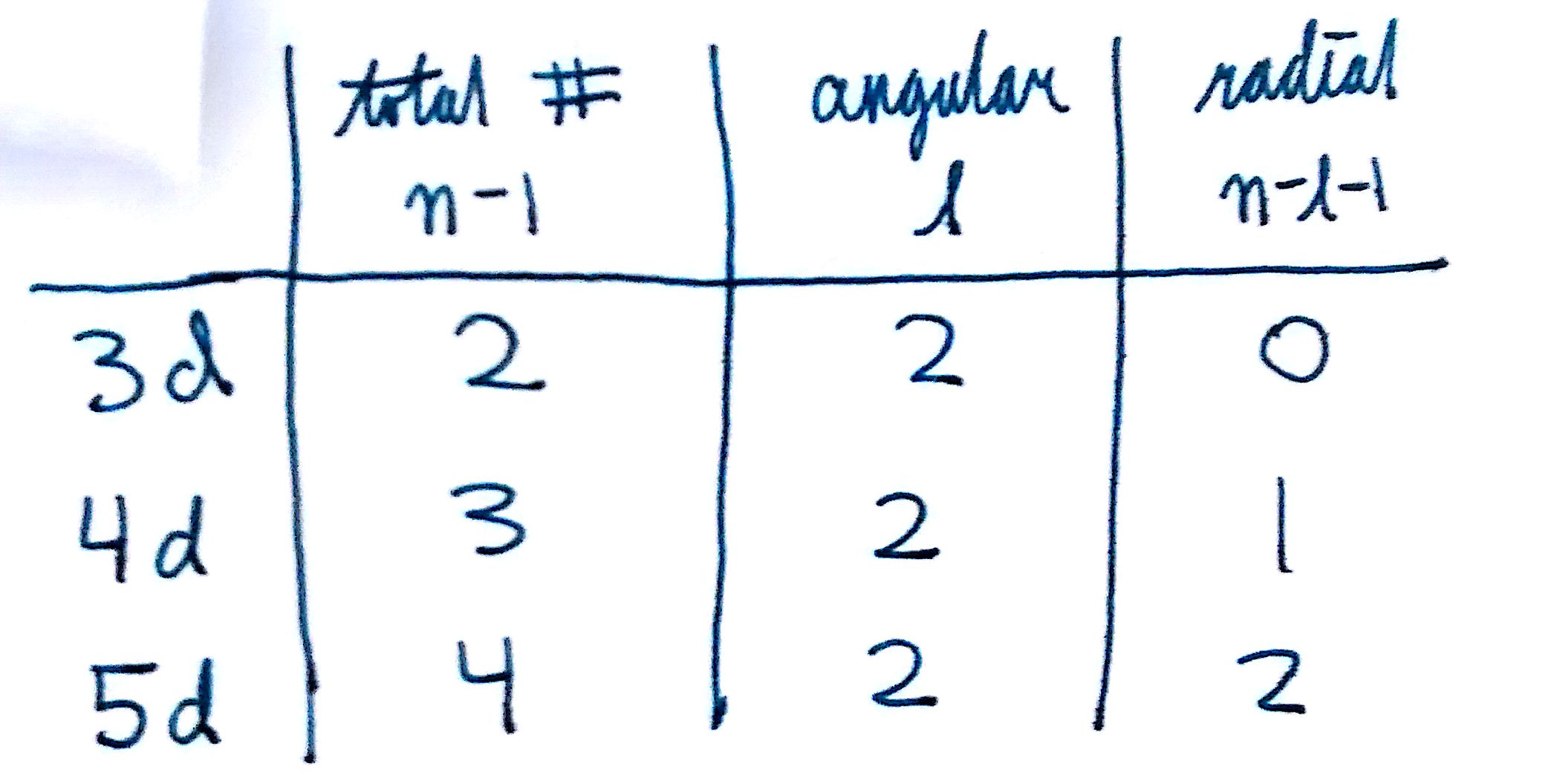
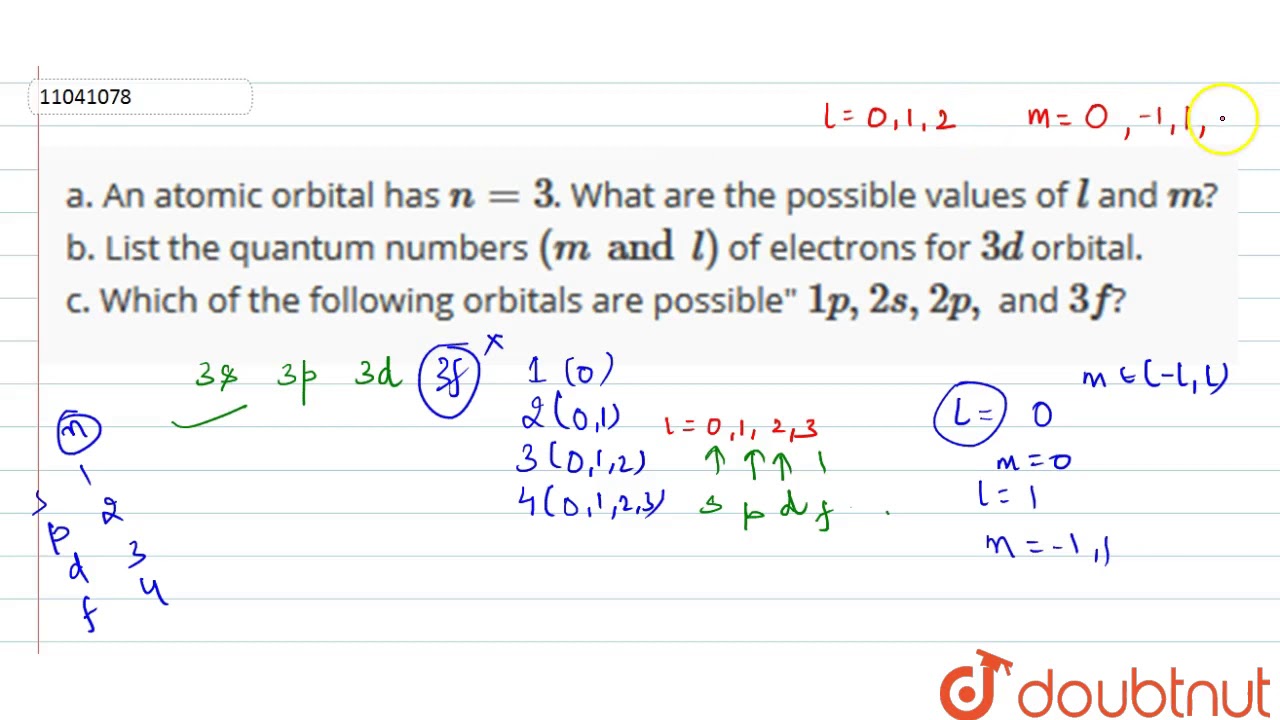
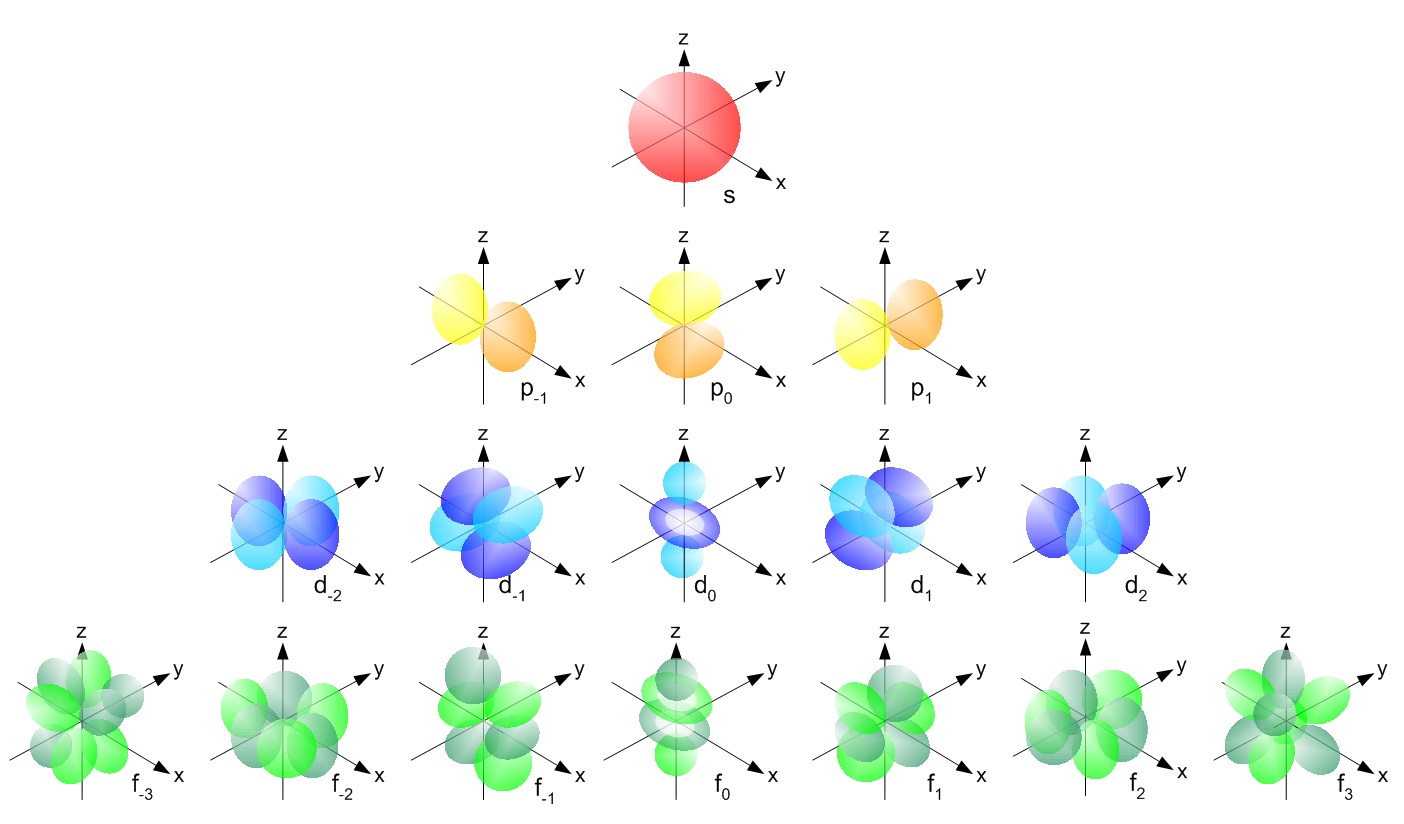
3d orbital quantum numbers. These numbers were first discovered in spectroscopy when the gaseous elements were exposed to a magnetic field. N 1 2 3. All three 2p orbitals or all five 3d orbitals where each orbital is occupied by an electron or each is occupied by an electron pair then all angular dependence disappears. The third quantum number is the magnetic quantum number m.
The atomic orbital is 3d. The three quantum numbers n l and m that describe an orbital are integers. So the value of principal quantum number for. Also in 1927 albrecht unsoeld proved that if one sums the electron density of all orbitals of a particular azimuthal quantum number of the same shell n eg.
The s correlates to 0 p to 1 d to 2 and f to 3. The principal quantum number n cannot be zero. 0 1 2 3 and so on. L is 1 for s electron 2 for p and 3 for d electron.
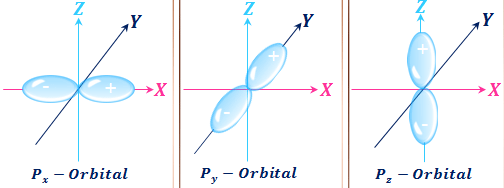
The allowed values of nare therefore 1 2 3 4 and so on. The angular momentum quantum number can be used to give the shapes of the electronic orbitals. The first three n l m l specify the particular orbital of interest and the fourth m s specifies how many electrons can occupy that orbital. The number of shells represents the principal quantum number.
See full answer below. In above problem it is 3d electron hence l for this is 3. The spectral line corresponding to a particular orbit would split into multiple lines when a magnetic field would be introduced across the gas. To determine which quantum numbers will correspond to an electron in a 3d orbital lets first define the values of first three quantum numbers.
The orbital letters are associated with the angular momentum quantum number which is assigned an integer value from 0 to 3. Each electron in an atom is described by four different quantum numbers. N for a 3d electron is 3.