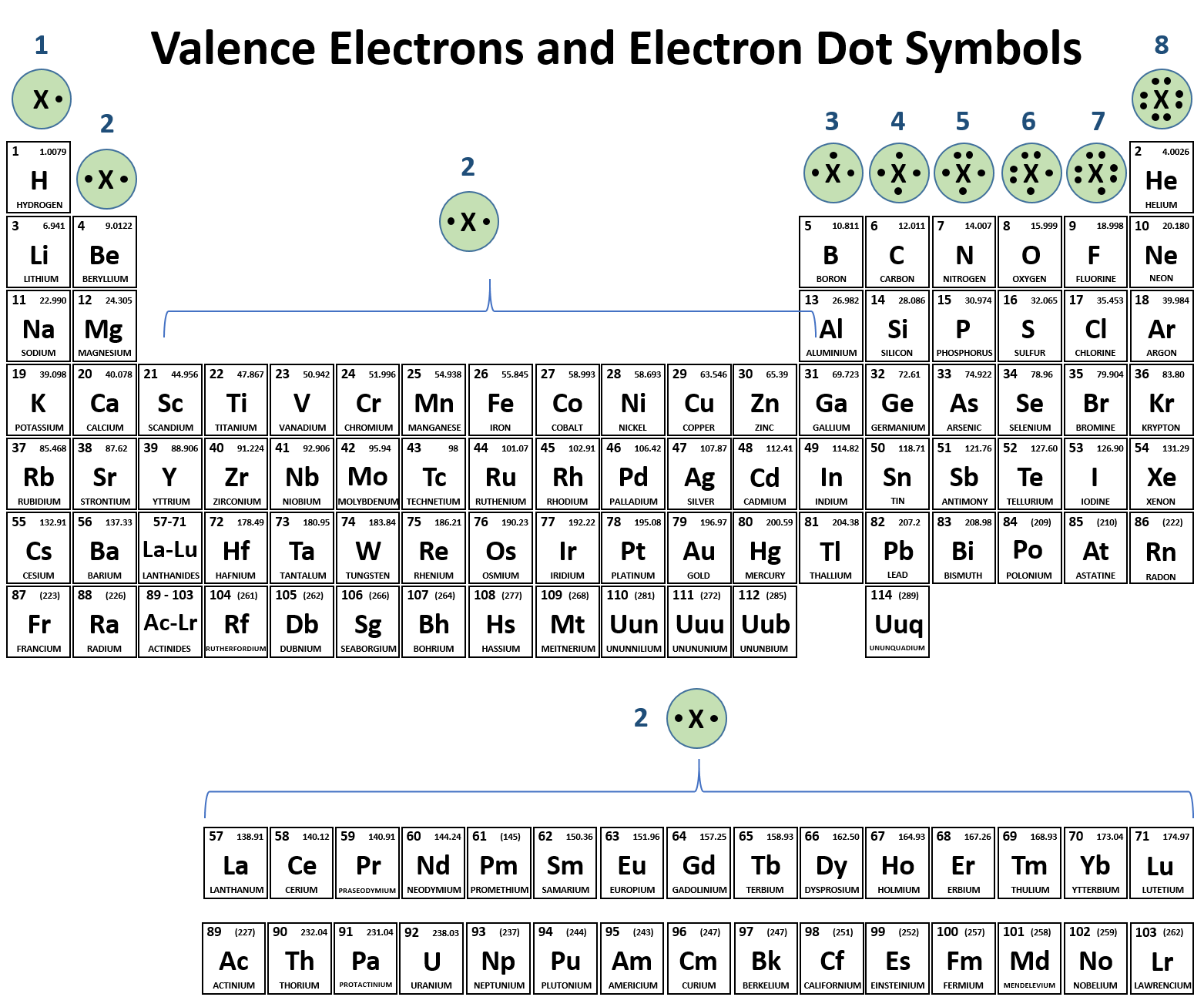
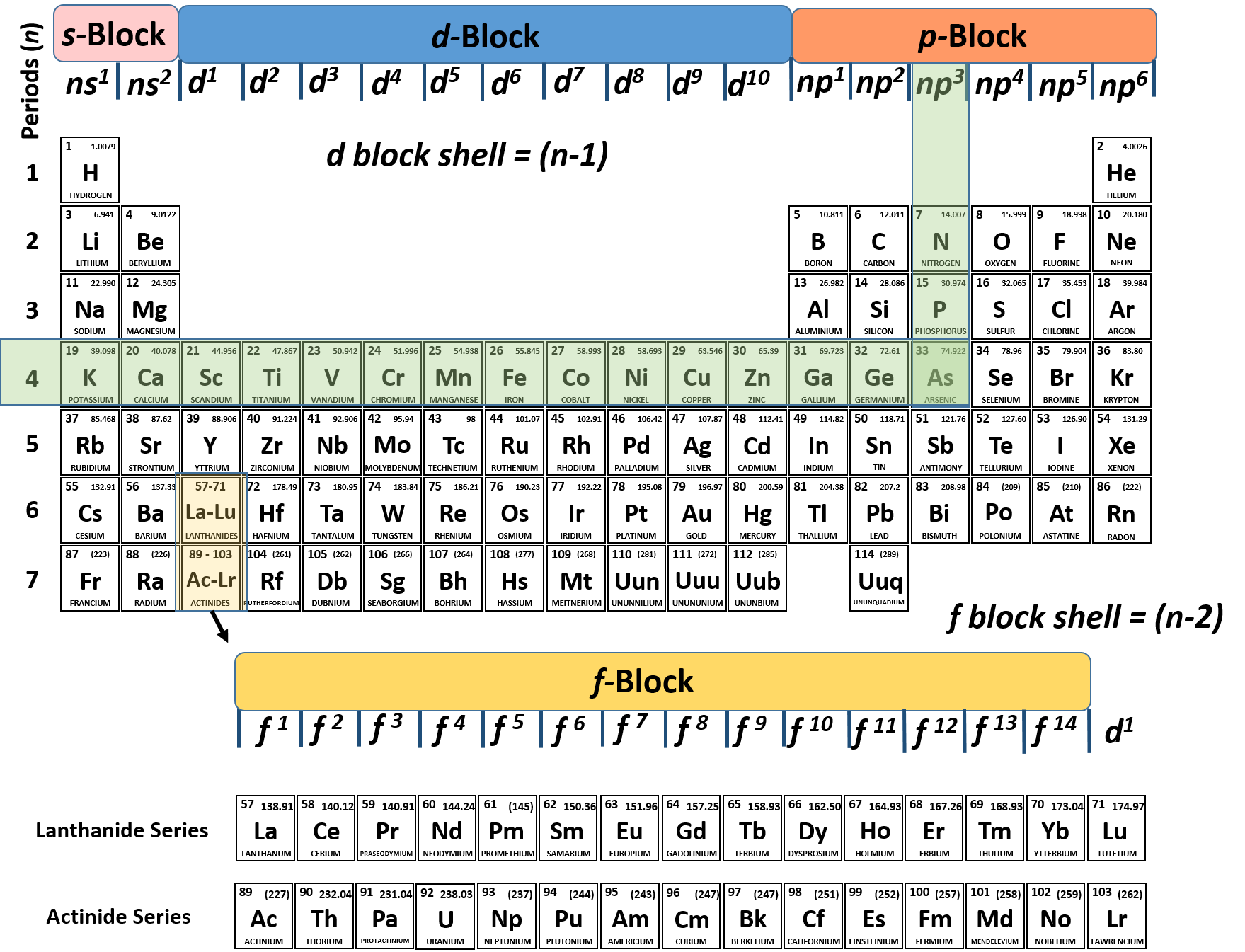
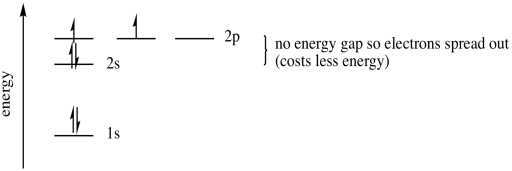Arsenic Does Not Have Any Valence Electrons In The 3d Orbital Because
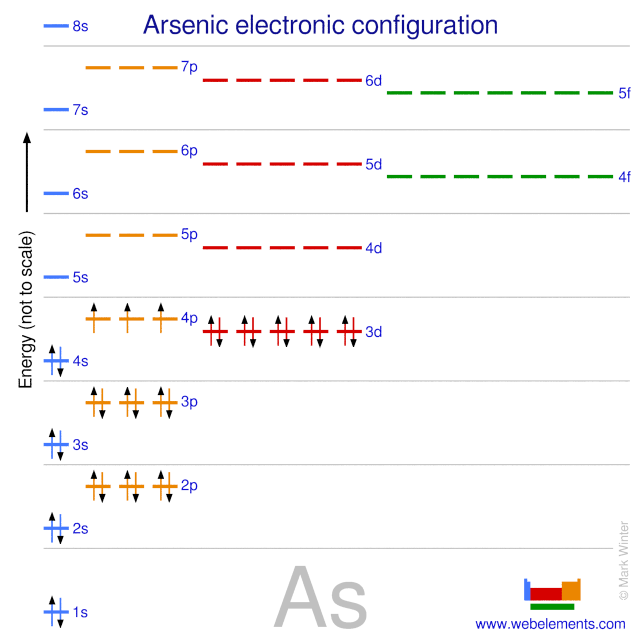
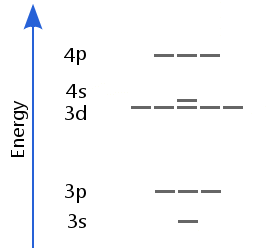
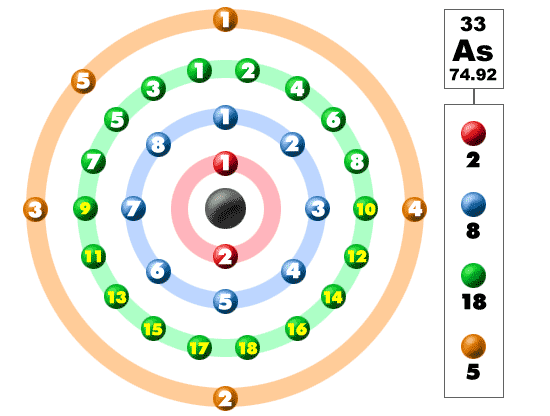
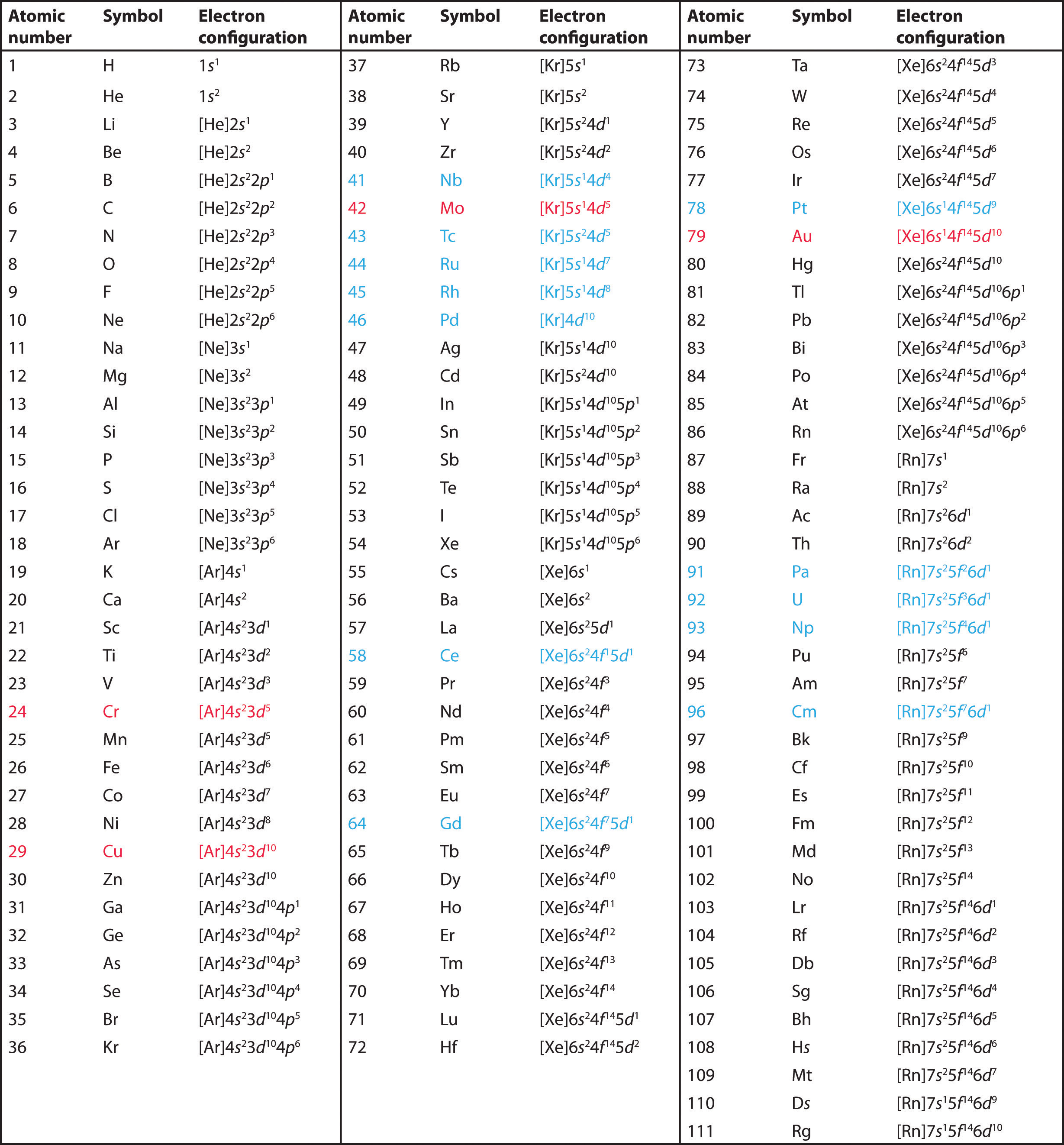
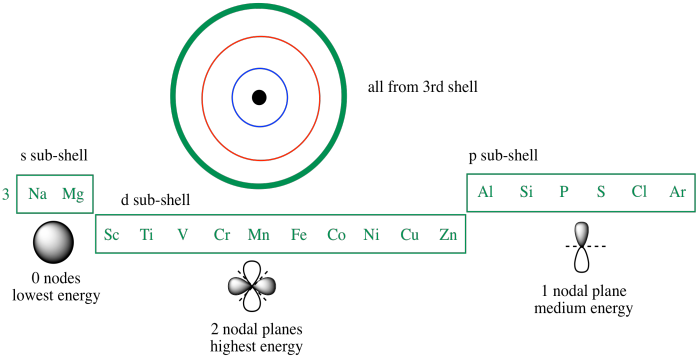
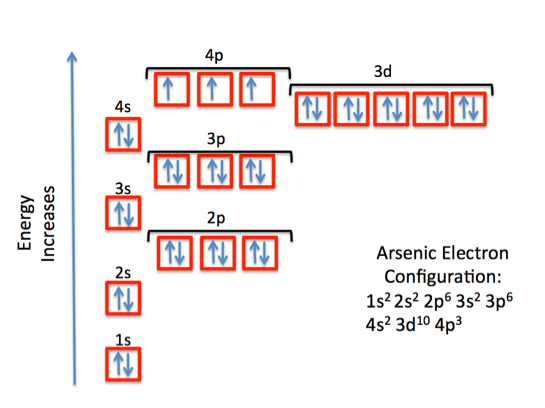
The electronic structure is ar 3d10.
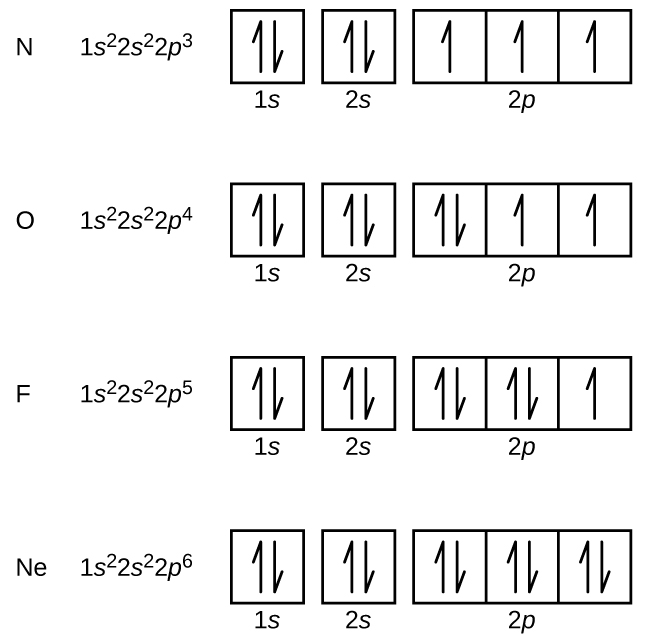
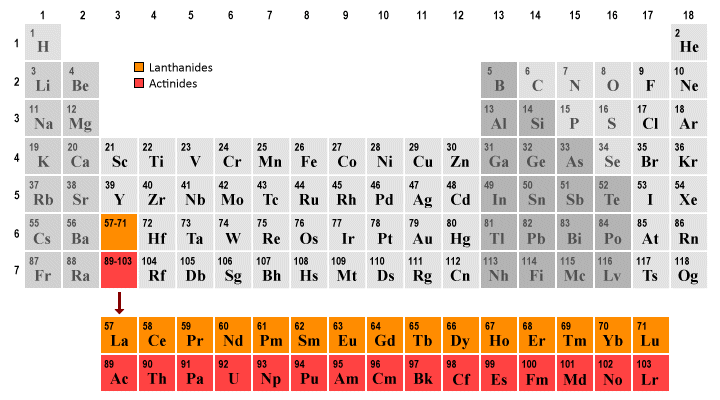
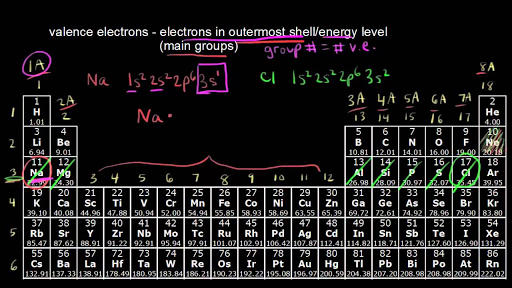
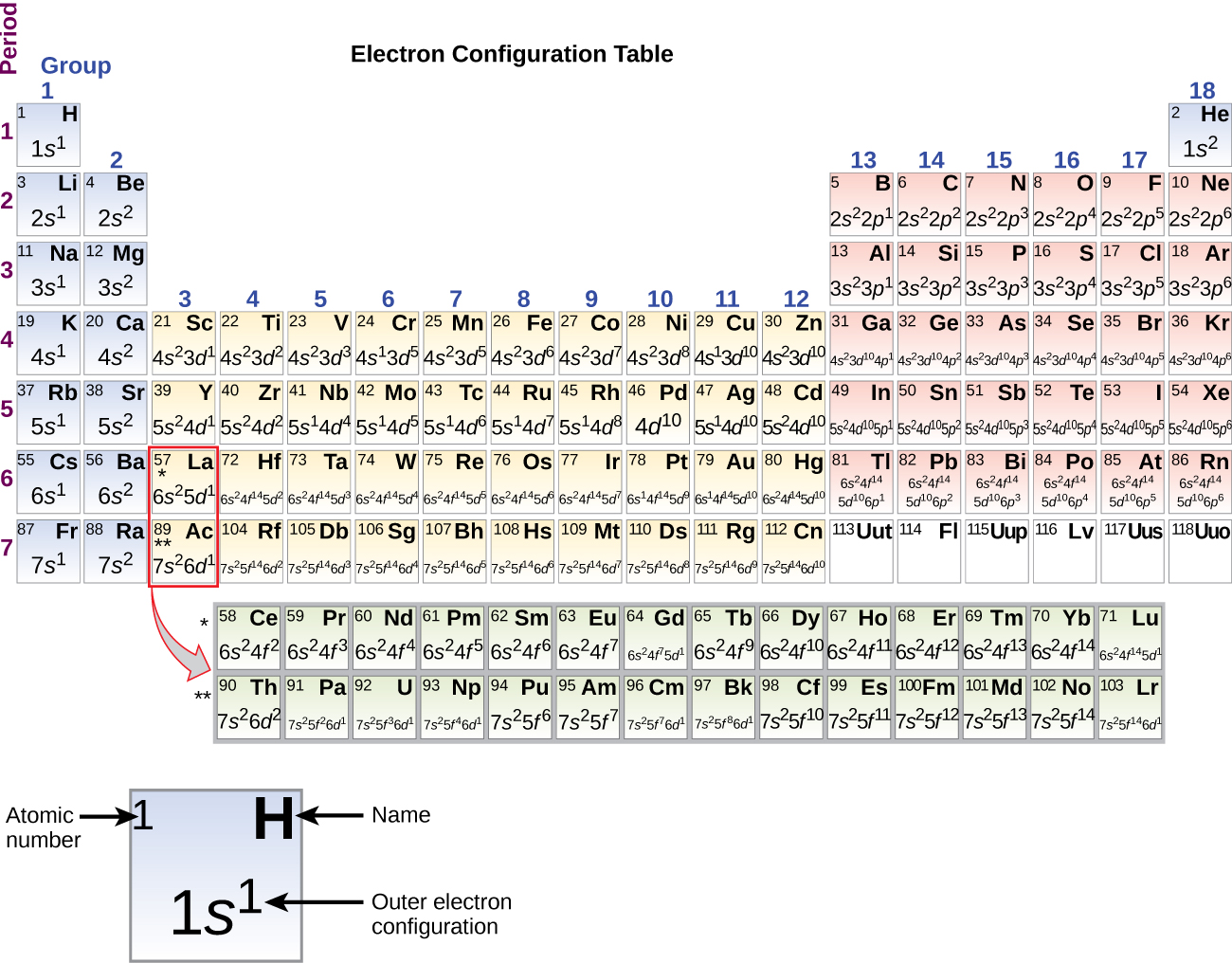
Arsenic does not have any valence electrons in the 3d orbital because. Arsenic does not have any valence electrons in the 3d orbital because its 3d orbital is completely filled. All you need is a periodic table. It is easier to determine the electron configurations for the p block elements in periods 1 2 and 3 than to determine the electron configurations for the rest of the p block elements in the periodic table because. Here is the trick.
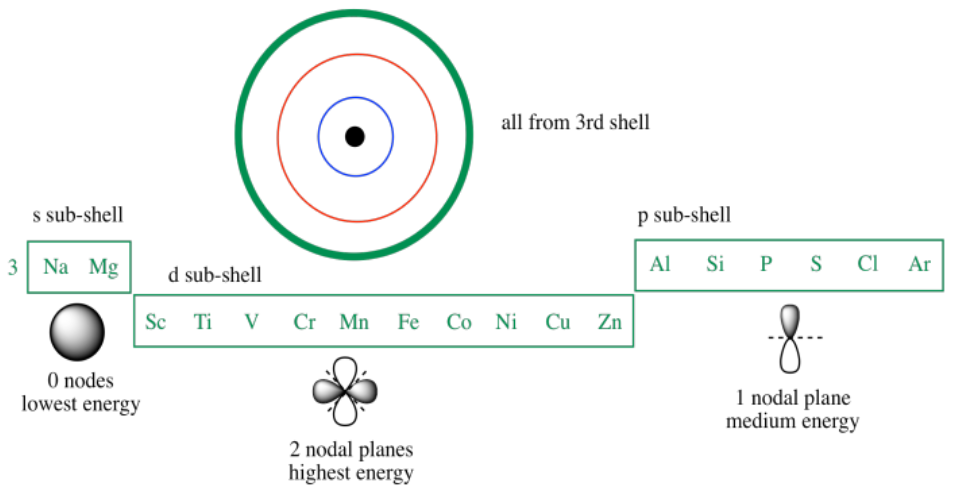
Id only count the s and p orbital electrons as valence electrons due to the d orbitals being filled. On other hand 3rd shell is not the valence shell in case of arsenic. It only contains enough electrons to fill the 2s orbital. An orbital can contain two electrons only if the electrons have opposite which model allows a student to determine the total number of electrons in an atom and the electrons within arsenic does not have any valence electrons in the 3d orbital because a its 3d orbital is completely.
Pick a picture of a periodic table. It is an inert substance that is not reactive. That leaves the two 4s and the three 4p orbital electrons as valence electrons. Arsenic is in period 4 so valence electrons will be in 4th shell.
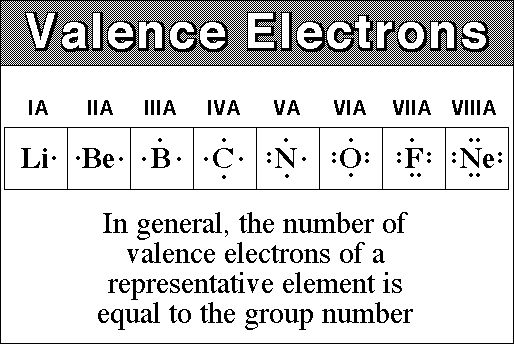
Now valence electrons are the electrons present in outermost shell. All of its valence electrons are in the 3p orbital. Arsenic has the same valence electron structure as nitrogen since its in the same group on the periodic table. Arsenic does not have any valence electrons in the 3d orbital because its 3d orbital is completely filled.
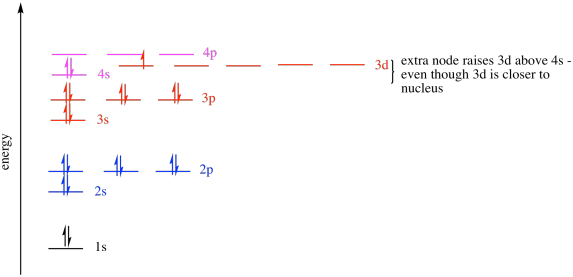
The group number that element is in will tell you how many electrons the element has. It is a p block element so filling orbitals will be p 4p and it comes after transition elements so it will have the 3d. Hence electrons present of 3d shell of arsenic cannot be referred as valence electrons. With reference to arsenic valence shell is n 4.
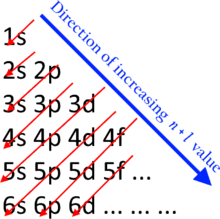
And in 4th shell there are no electrons present in d orbital. It only contains enough electrons to fill the 2s orbital. I will assume your question refers to why the 3d electrons of arsenic are not included as valence electrons since they populate the shells of the atom after the 4s electrons which are valence electrons. Electrons fill orbitals in order of their increasing energy from left to right.