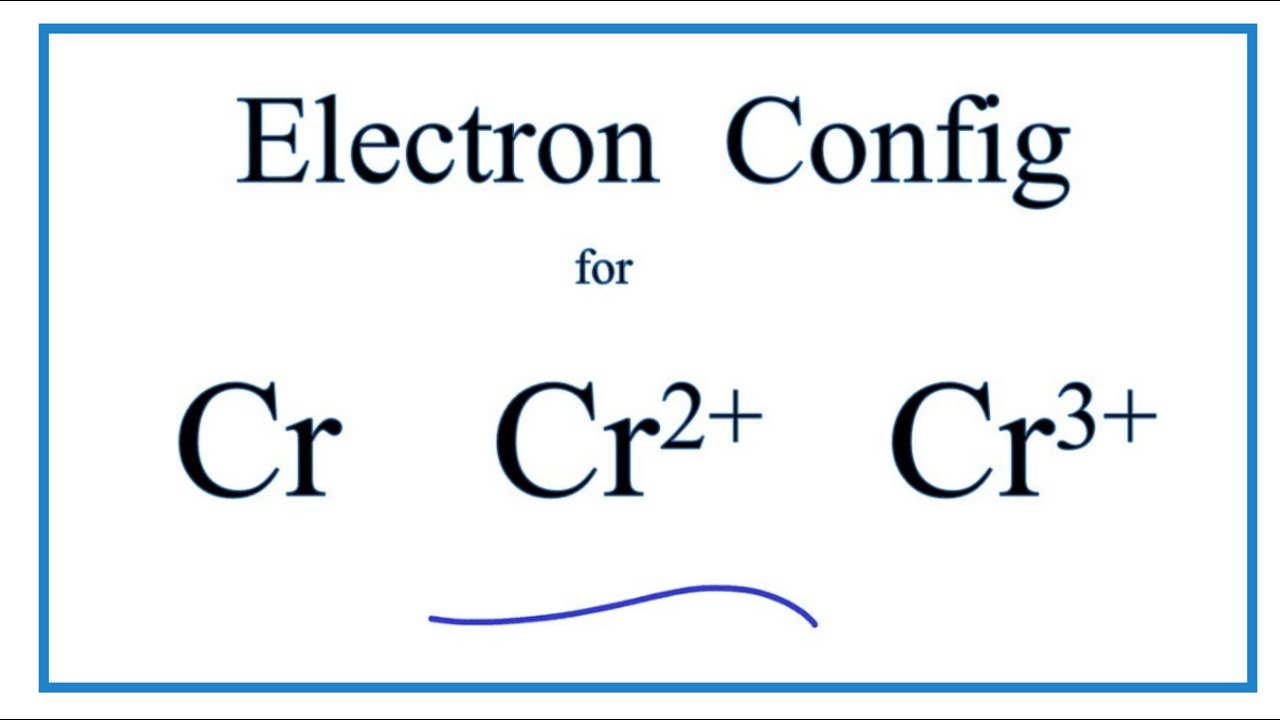
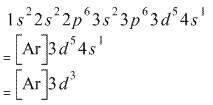The Total Number Of Electrons In The 3d Orbitals Of Cr3 Is
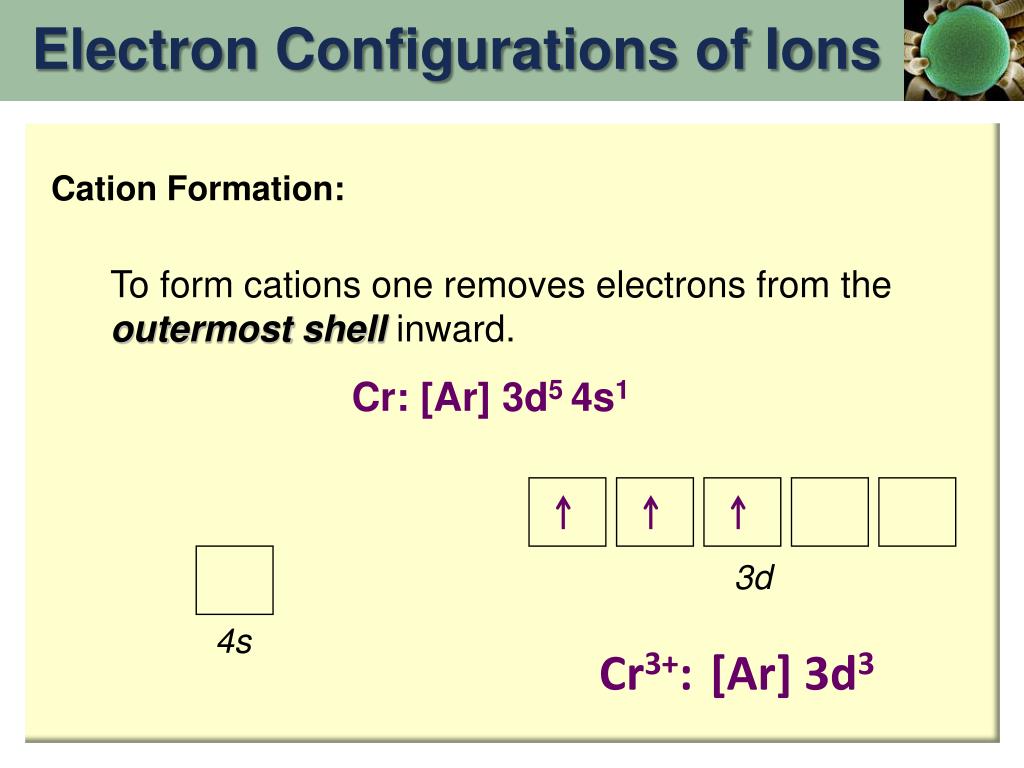
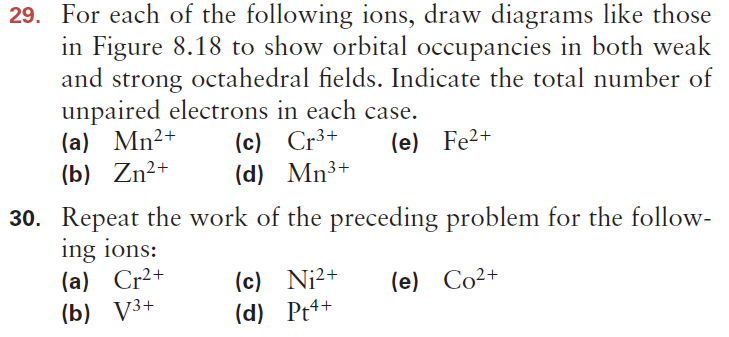
So that if it lost an electron it becomes a cation cr and his electronic.
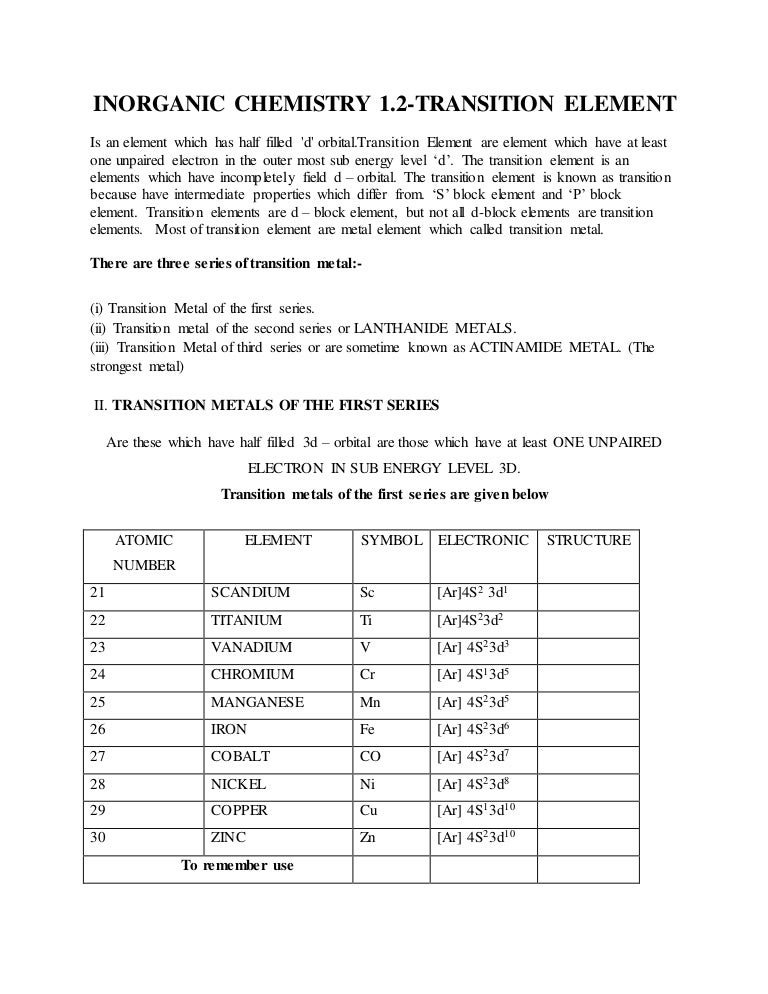
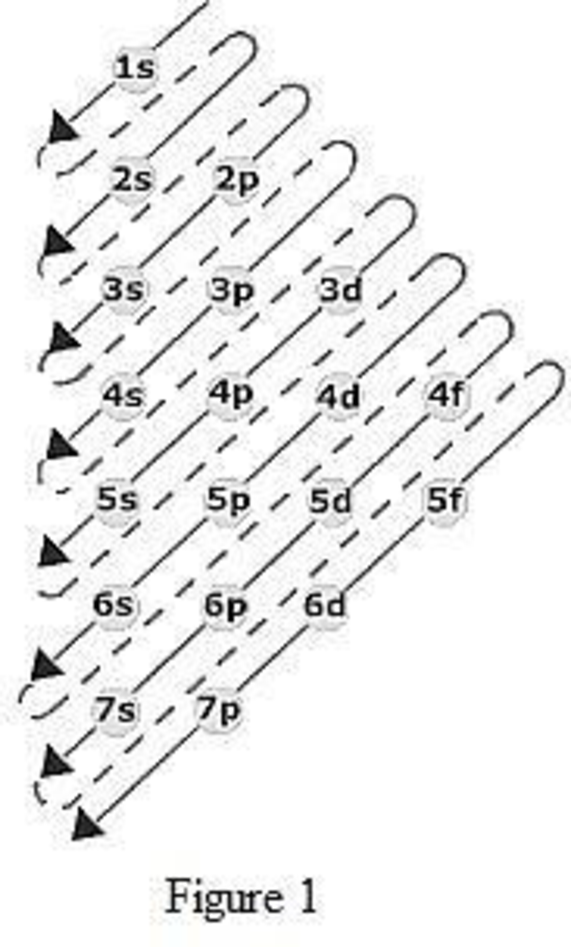
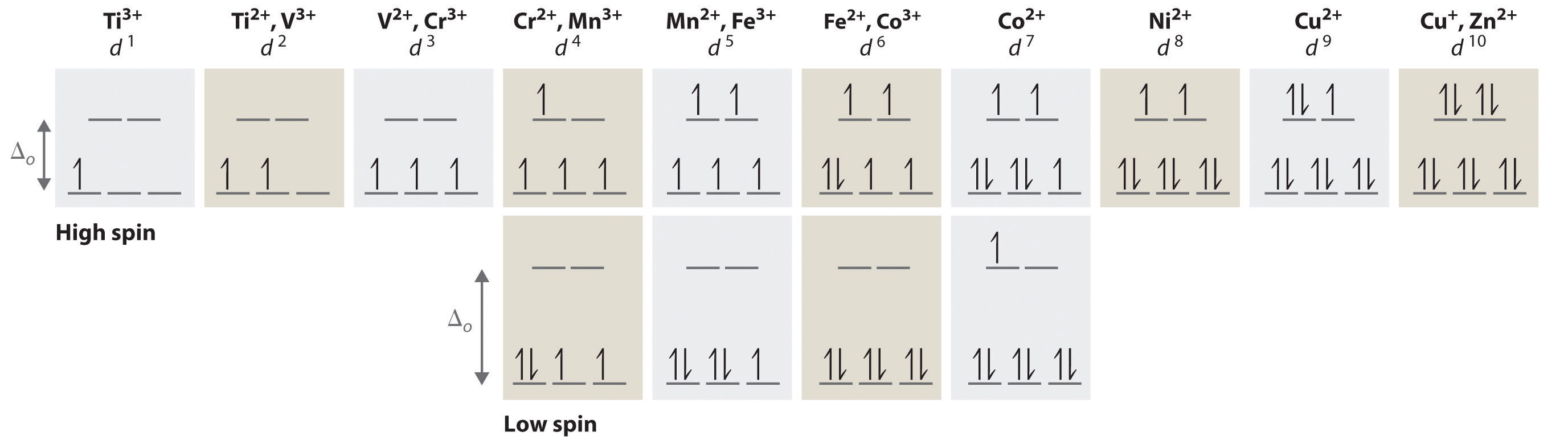
The total number of electrons in the 3d orbitals of cr3 is. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 3. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Total number of electrons in 3d orbital of cr3 plus is answers. Fill orbitals until you reach 21 electrons cr3 has 21 electrons so its configuration should.
This problem has been solved. Please also provide an explanation. In cr3 ion there are only three electrons because duringoxidation one from 4s orbital and two from 3d orbitals have beenlost. Chemistry questions and answers the total number of electrons in the 3d orbitals of cr3 is a.
The total number of electrons in the 3d orbitals of a copper atom is a 6 b 7 c8 d 9 e 10. The total number of electrons in the 3d orbitals of a copper atom is a 6 b 7 c8 d 9 e 10. 1s2 2s2 2p6 3s2 3p6 3d5 4s1. For the cr 3 ion we remove a total of three electrons one from the 4s1 and two from the 3d5 leaving us with.