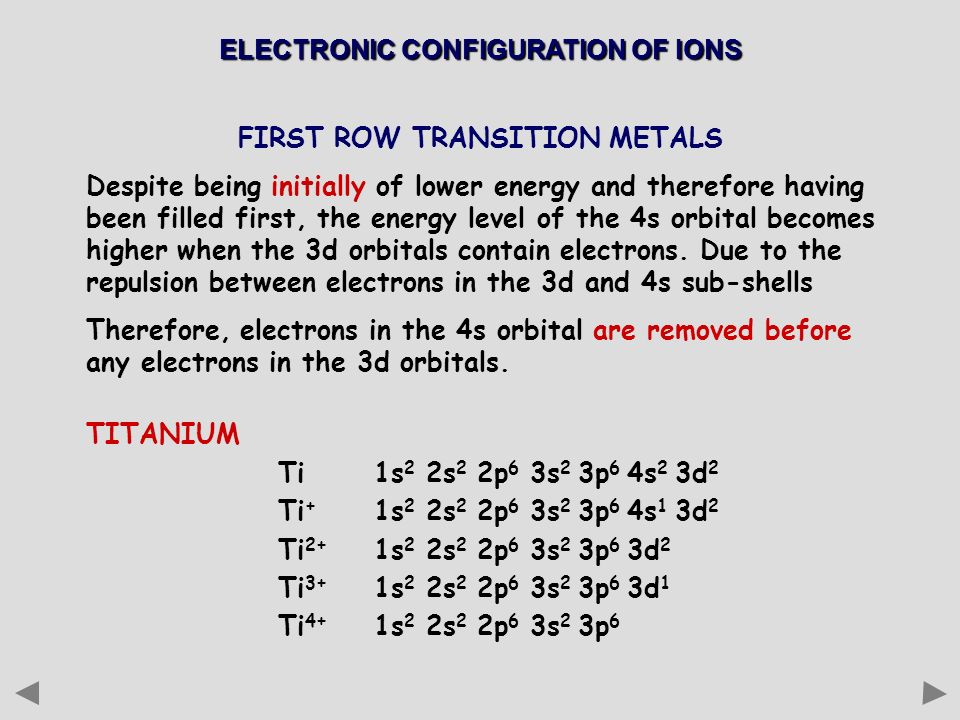
The Total Number Of Electrons In The 3d Orbitals Of Ti3 Is
1s 2s 2p x 2p y and 2p zthe two colors show the phase or sign of the wave function in each region.
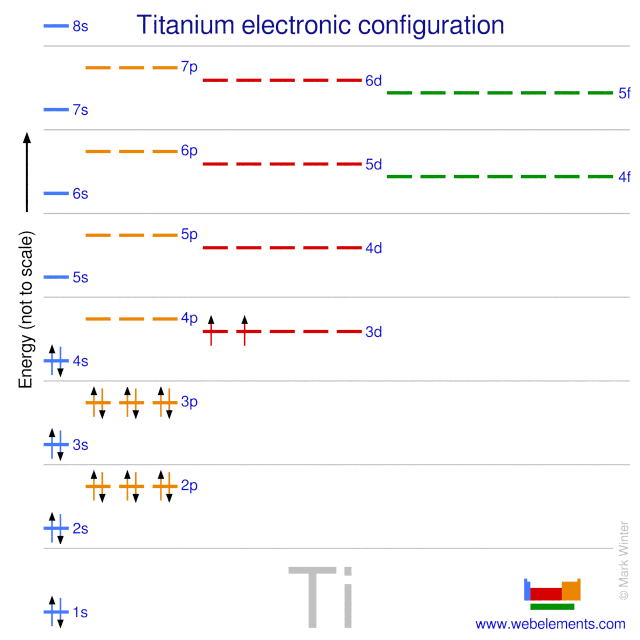
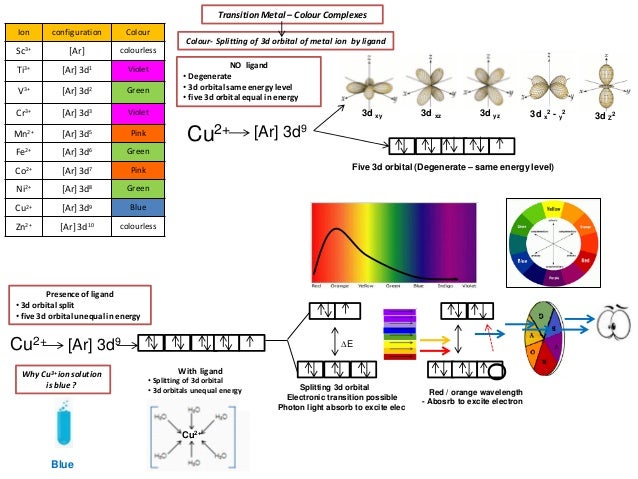
The total number of electrons in the 3d orbitals of ti3 is. Ar4s2 3d2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d2. The total number of electrons in the 3d orbitals of a titanium atom is. This problem has been solved. The d electron count is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex.
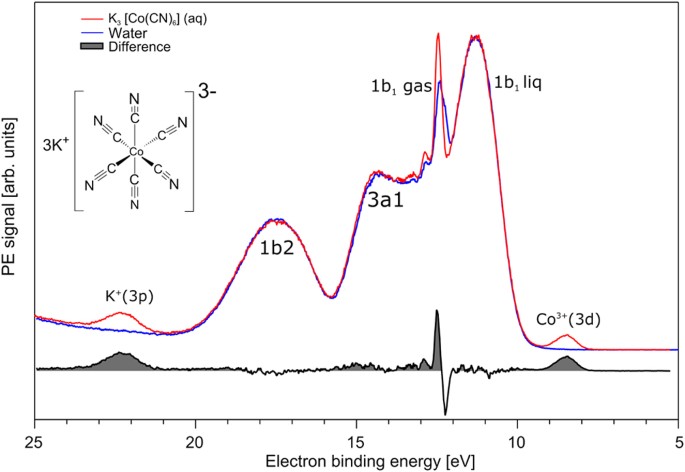
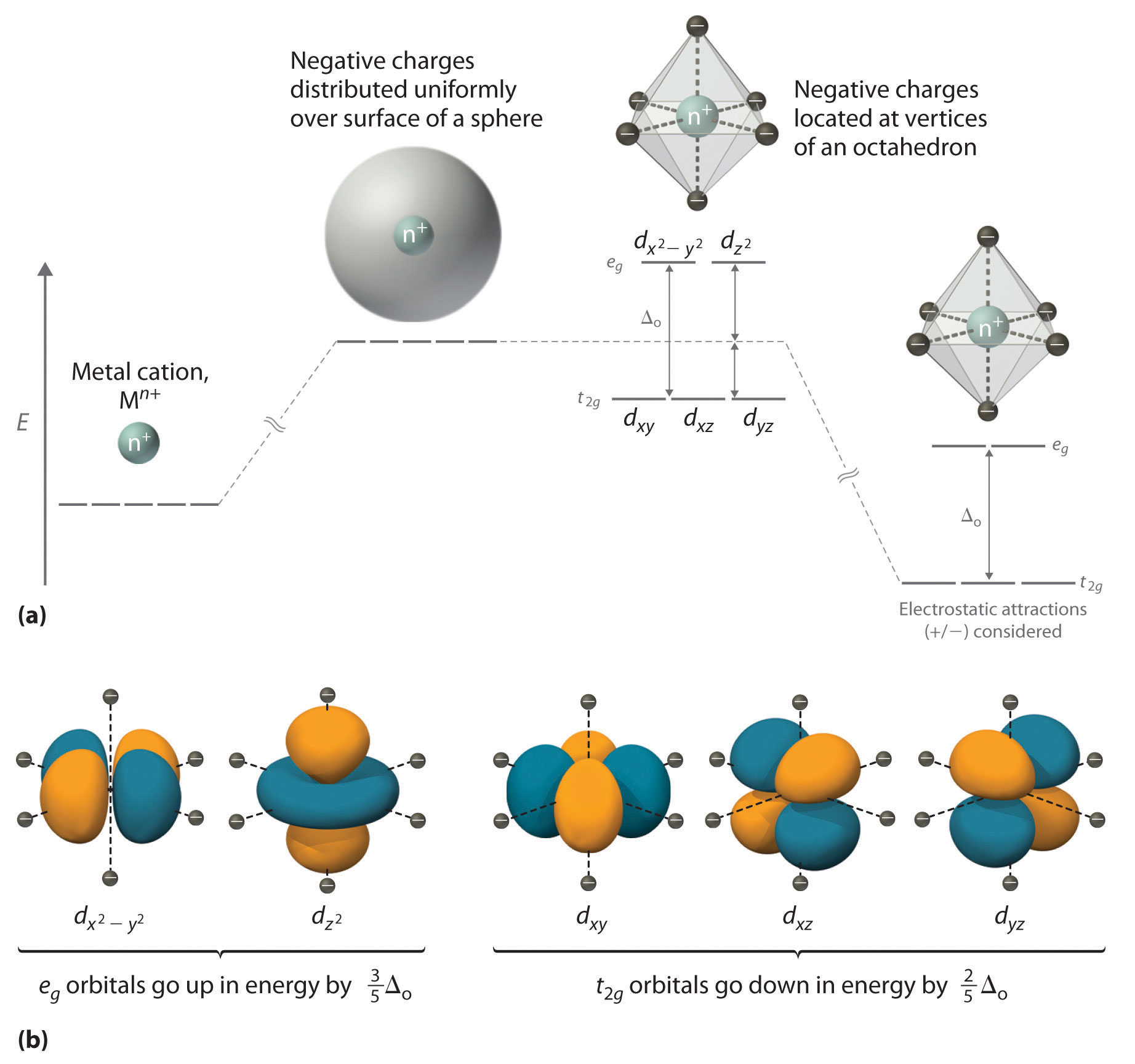
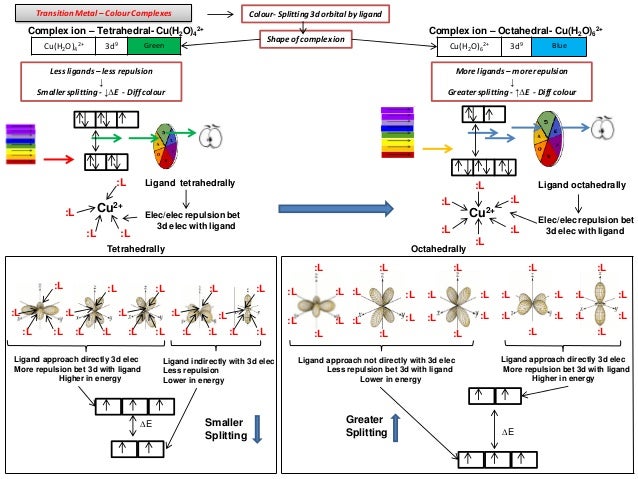
The shapes of the first five atomic orbitals are. The total number of electrons in the 3d orbitals of a copper atom is a 6 b 7 c8 d 9 e 10. Each picture is domain coloring of a psx y z function which depend on the coordinates of one electron. The characteristics of transition metal ligand bonds become clear by an analysis of the molecular orbitals of a 3d metal coordinated by six identical ligands in octahedral complexes ml 6as the result of the interaction between the metal d and ligand orbitals bonding non bonding and anti bonding complex molecular orbitals are formed.
To see the elongated shape of psx y z 2 functions that show probability density more directly see pictures of d orbitals below. Molecular orbital theory of transition metal complexes. The total number of electrons in the 3d orbitals of a copper atom is a 6 b 7 c8 d 9 e 10. The total number of electrons in the 3d orbitals of a titanium atom is.
The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes. The formalism has been incorporated into the two major models used to describe coordination complexes. Get 11 help now from expert chemistry tutors. And therefore the d suborbital has 2 electrons in.
Previous question next question get more help from chegg.