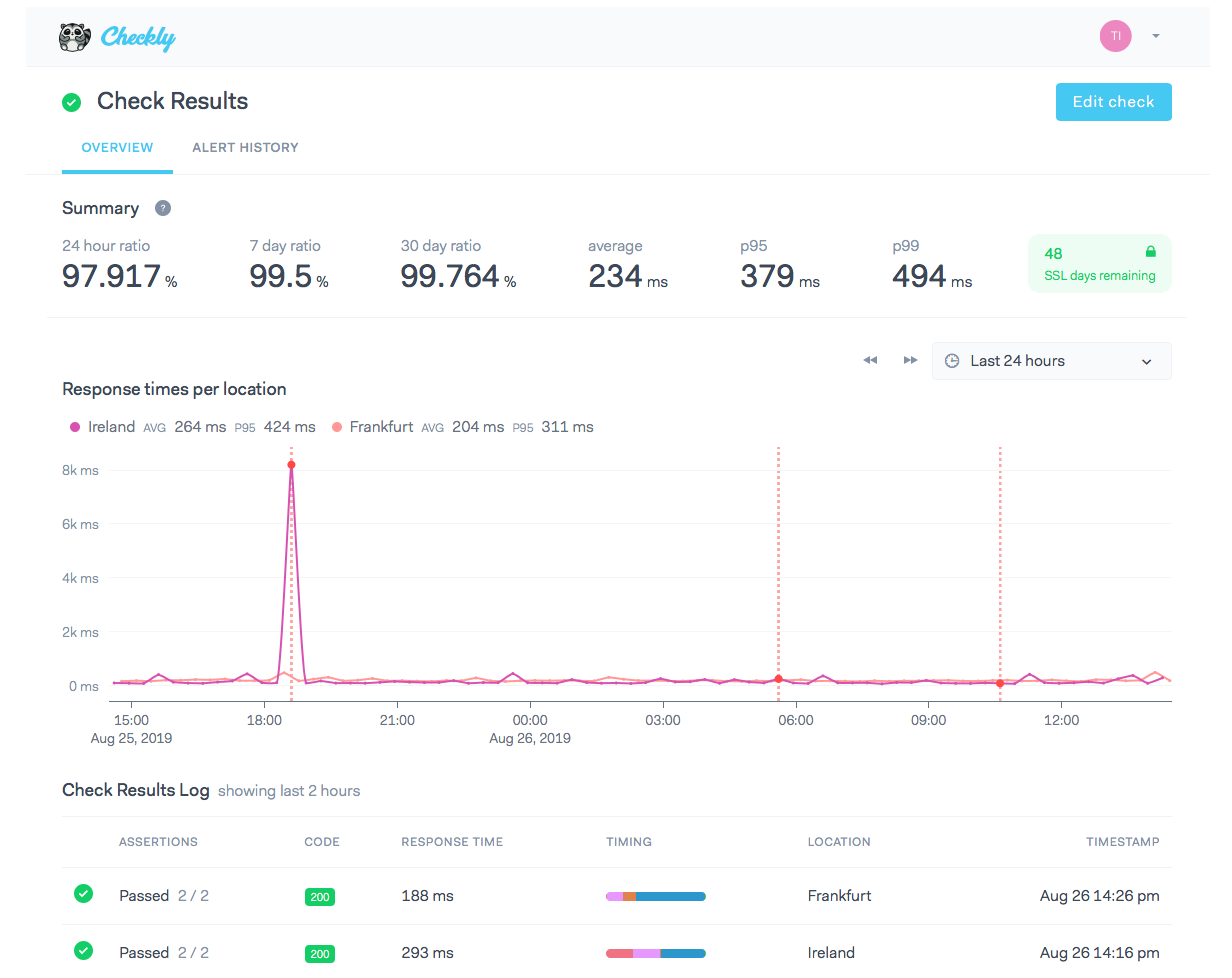
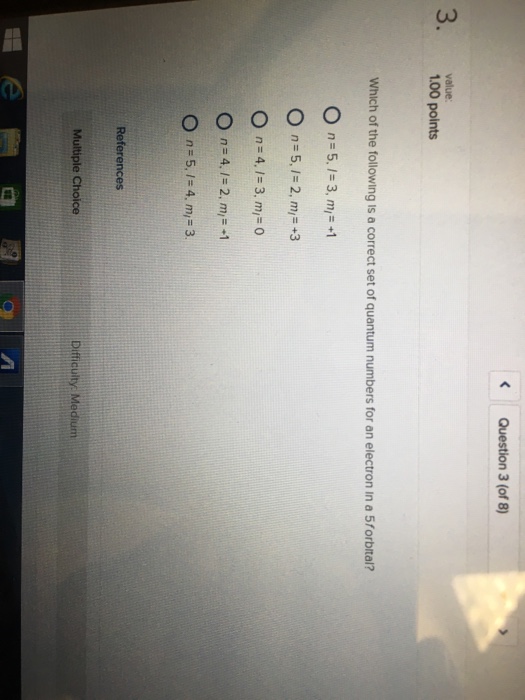
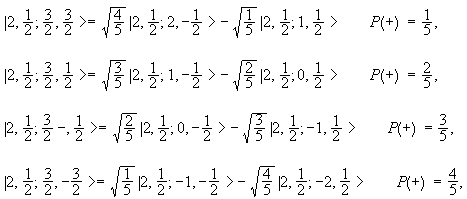Which Of The Following Is A Correct Set Of Quantum Numbers For An Electron In A 3d Orbital Chegg
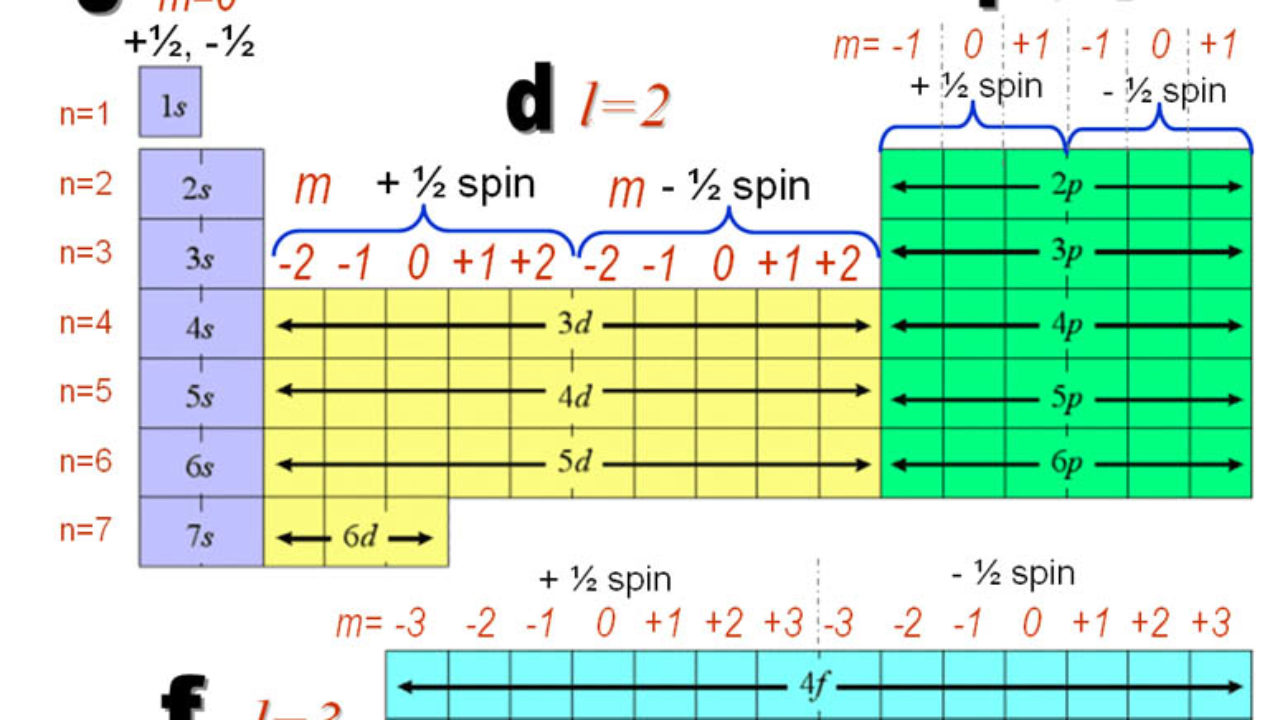
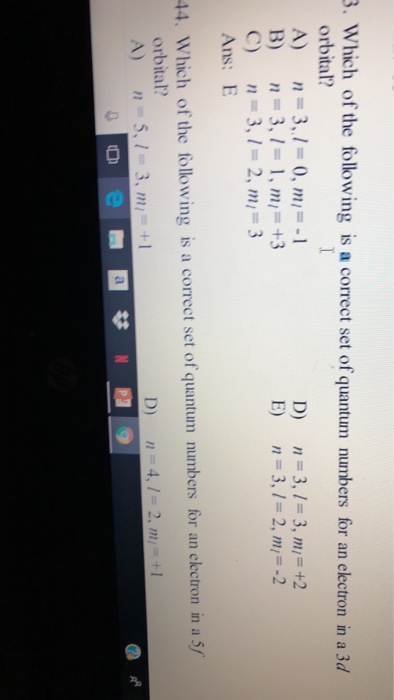
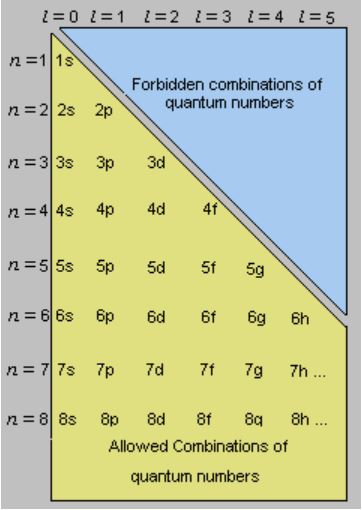
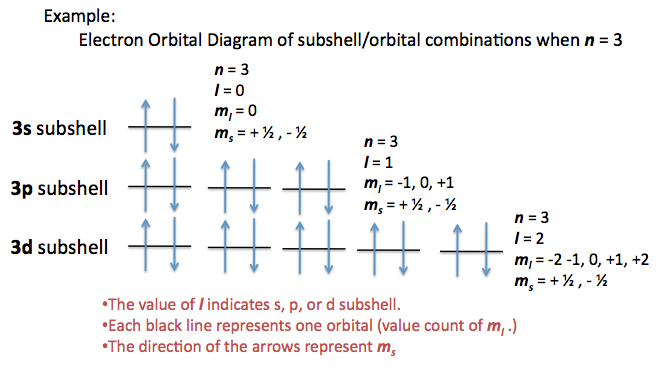
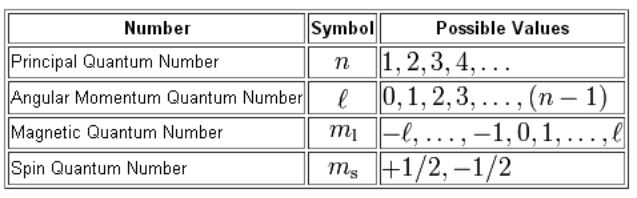
N for a 3d electron is 3.
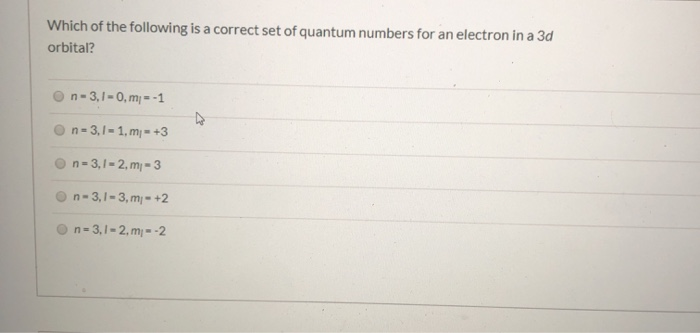
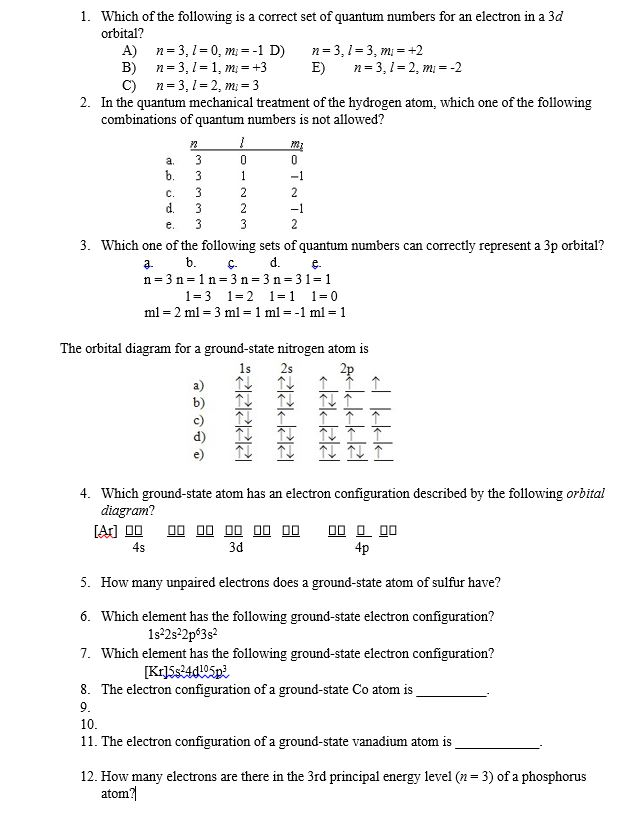
Which of the following is a correct set of quantum numbers for an electron in a 3d orbital chegg. L is 1 for s electron 2 for p and 3 for d electron. Which one of the following sets of quantum numbers can correctly represent a 3p orbital. Each electron in an atom must have its own unique set of quantum numbers is one way to state a the aufbau principle. Mi3 dn31 3 mi 2 e.
Each electron has its unique set of quantum numbers which means that two electrons can share one two or even three quantum numbers but never all four. In above problem it is 3d electron hence l for this is 3. The energy of an electron in an atom is quantized 2. For chlorine atom z 1 7 the last electron enters into 3p orbital for which n 3 l 1 m 1 0 or 1 and s 2 1.
The s value can be 12 or 12. An31 0 mi 1 bn31 1. Which of the following is a correct set of quantum numbers for an electron in a 3d orbital. Therefore the correct set of the four quantum numbers n3l2m2s12.
D n 2 l 3 ml 2 ms 12. Now you are given a colorred4d orbital and asked to find how many sets of. Which of the following isare correct postulates of bohrs theory of the hydrogen atom. C n 2 l 2 ml 2 ms 12.
N31 2 mi 2 answer the following questions. A n 3 l 2 ml 2 ms 12. Which is a correct set of quantum numbers for an electron in a 3d orbital. B n 3 l 2 ml 3 ms 12.
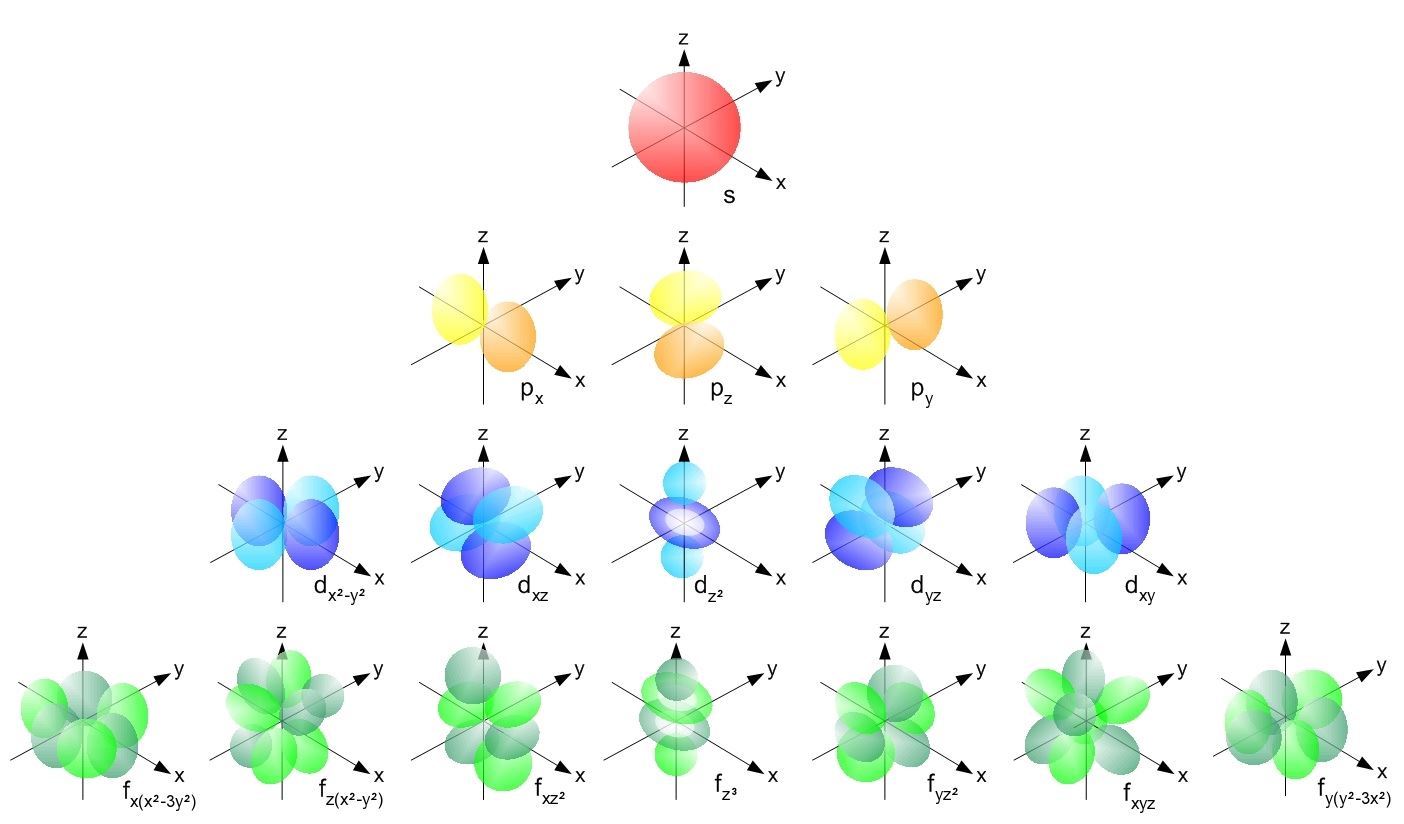
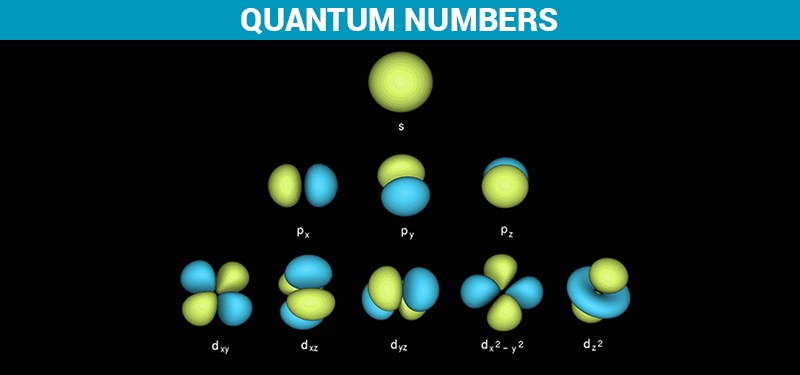
B the pauli exclusion principle. The d subshell represents the l value 2 whereas subshell d has 21012as the m values for the orbitals. Which of the following is the correct set of quantum numbers for an electron in a 3d orbital. Mi 3 con31 2.
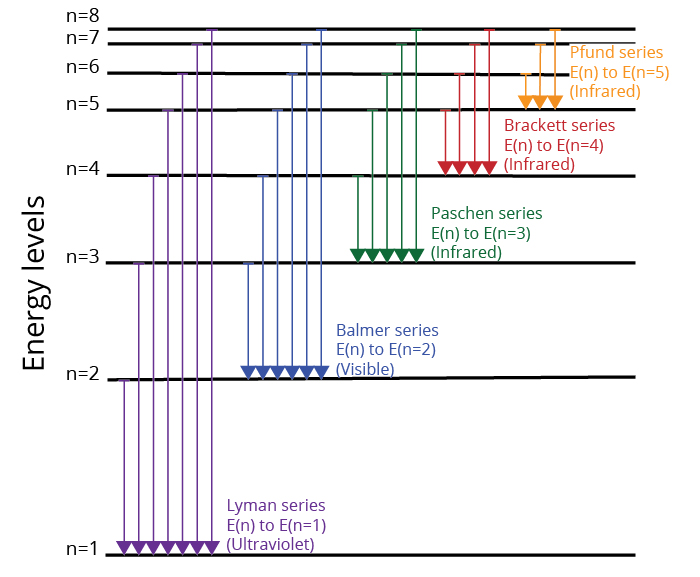
In the quantum mechanical treatment of the hydrogen atom which one of the following combinations of quantum numbers is not allowed. An electron transition from a lower energy level to a higher energy level results in an emission of a photon of light. As you know we use four quantum numbers to describe the position and spin of an electron in an atom. Therefore the 4f quantum level is filled with all of its electrons from atomic numbers 57 through 71 and only then is n 6p electron added to form an atom with atomic number 72a corresponding.
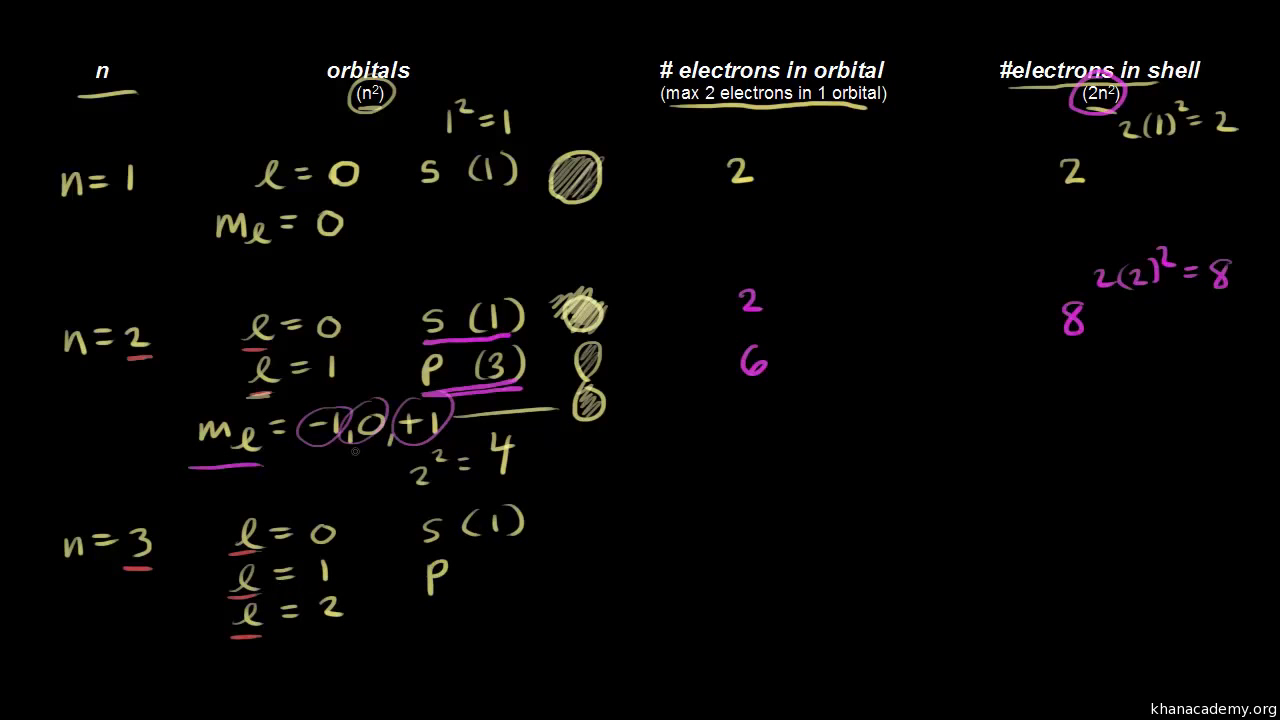
3d has 3 as the principal quantum number. A total of 10 sets of quantum numbers can be used here. The principal quantum number n specifies each unique energy level 3.