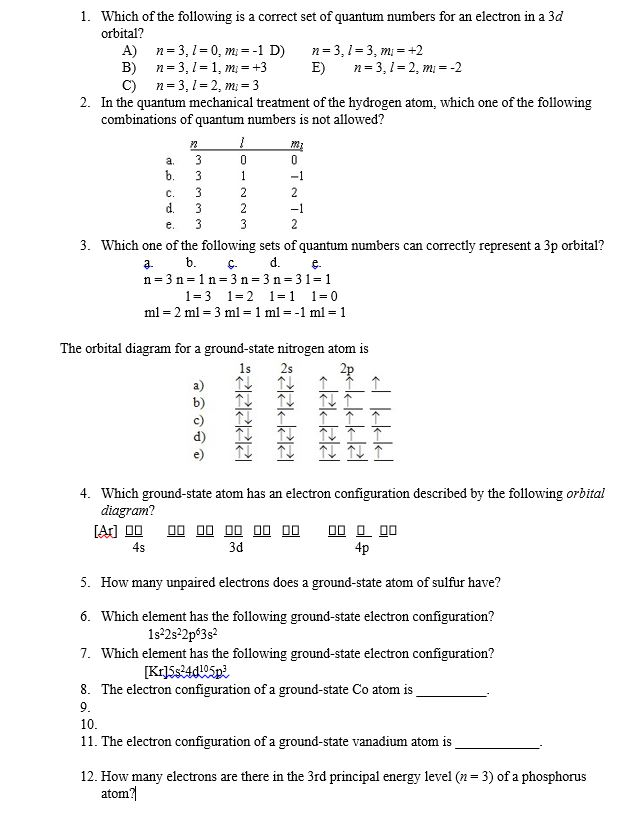
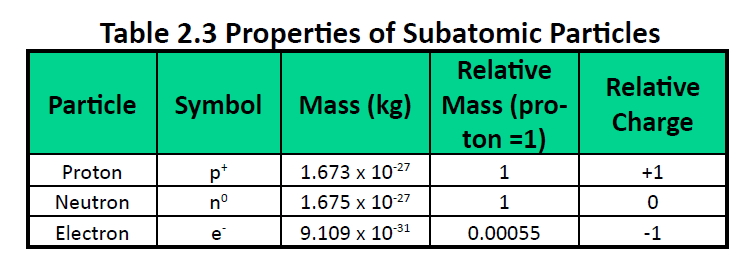
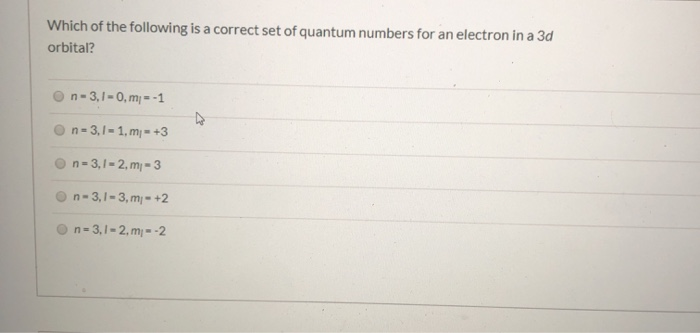
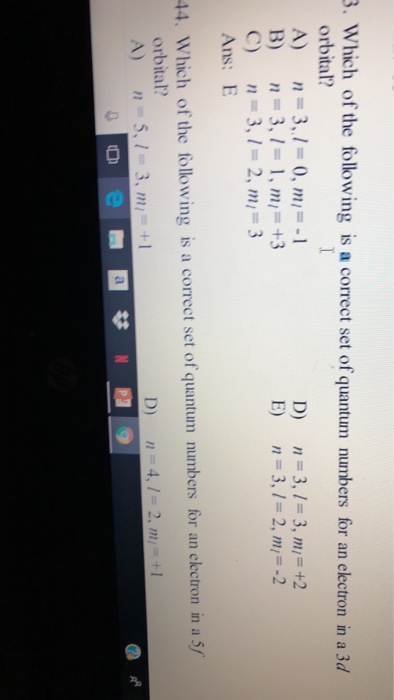
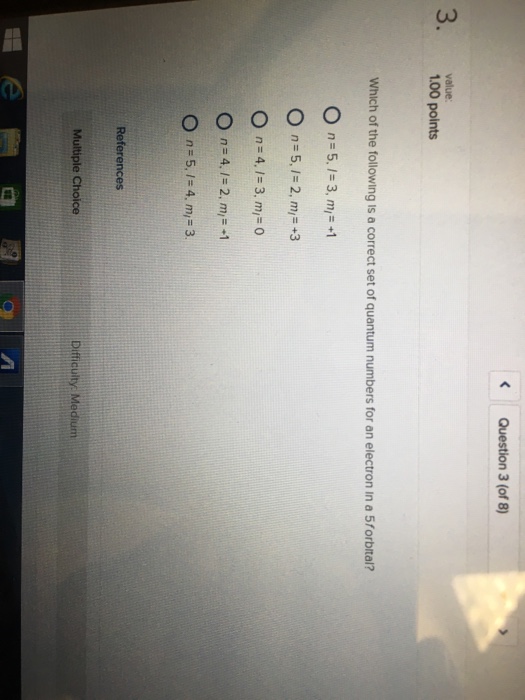
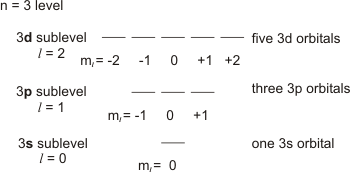Which Of The Following Is A Correct Set Of Quantum Numbers For An Electron In A 3d Orbital
3 2 1 12 what is the value for the angular momentum quantum number for the electron in a 5d orbital.

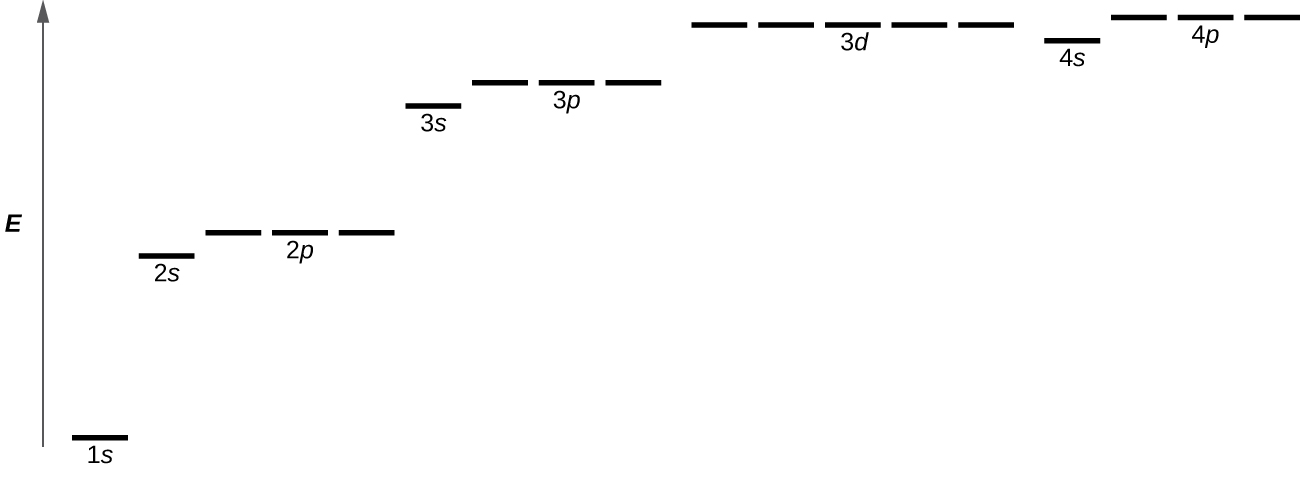
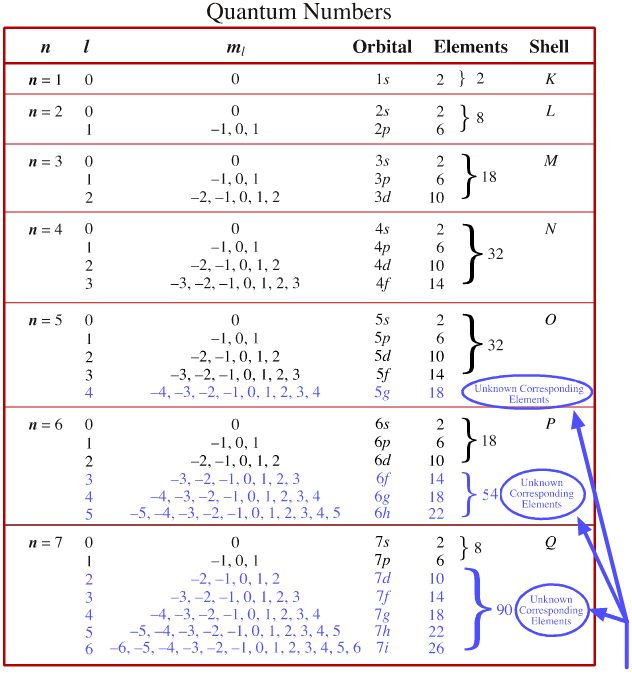
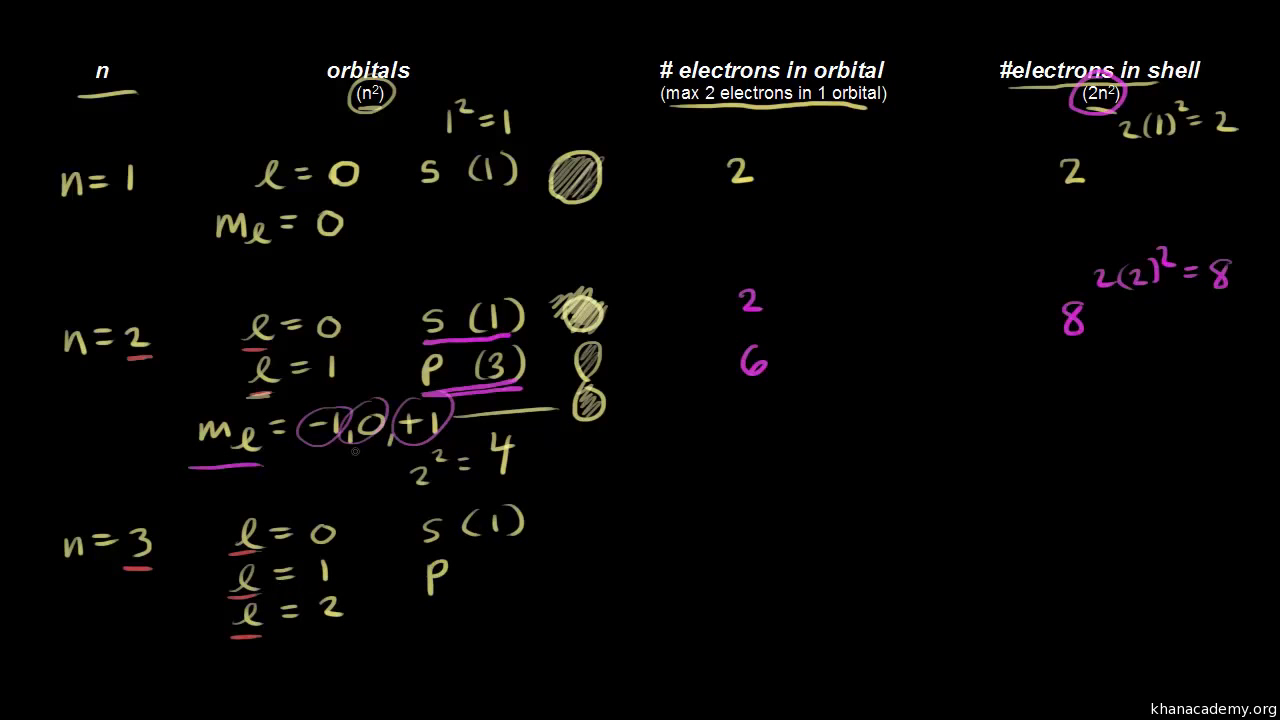
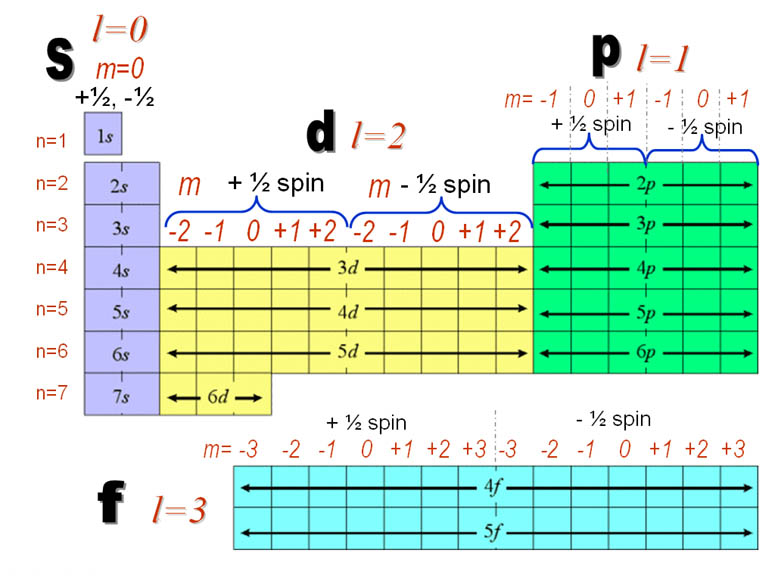
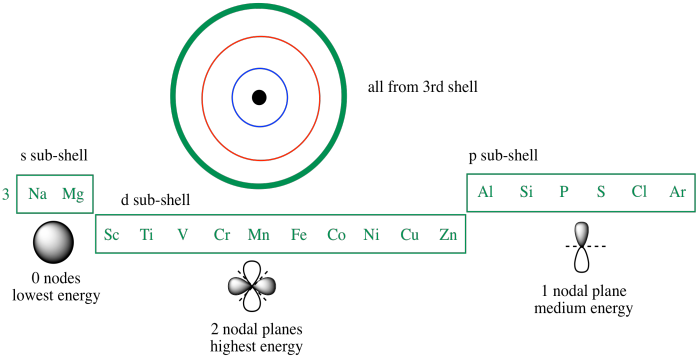
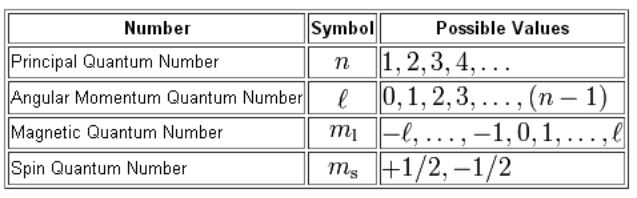
Which of the following is a correct set of quantum numbers for an electron in a 3d orbital. Which sets of quantum numbers is correct for an electron in 4f orbital atomic structure which of the following sets of quantum numbers is correct for an electron in 4f orbital. The distribution of electrons among the orbitals of an atom is called the electron configurationthe electrons are filled in according to a scheme known as the aufbau principle building up which corresponds for the most part to increasing energy of the subshells. The orbital diagram for. To determine which quantum numbers will correspond to an electron in a 3d orbital lets first define the values of first three quantum numbers.
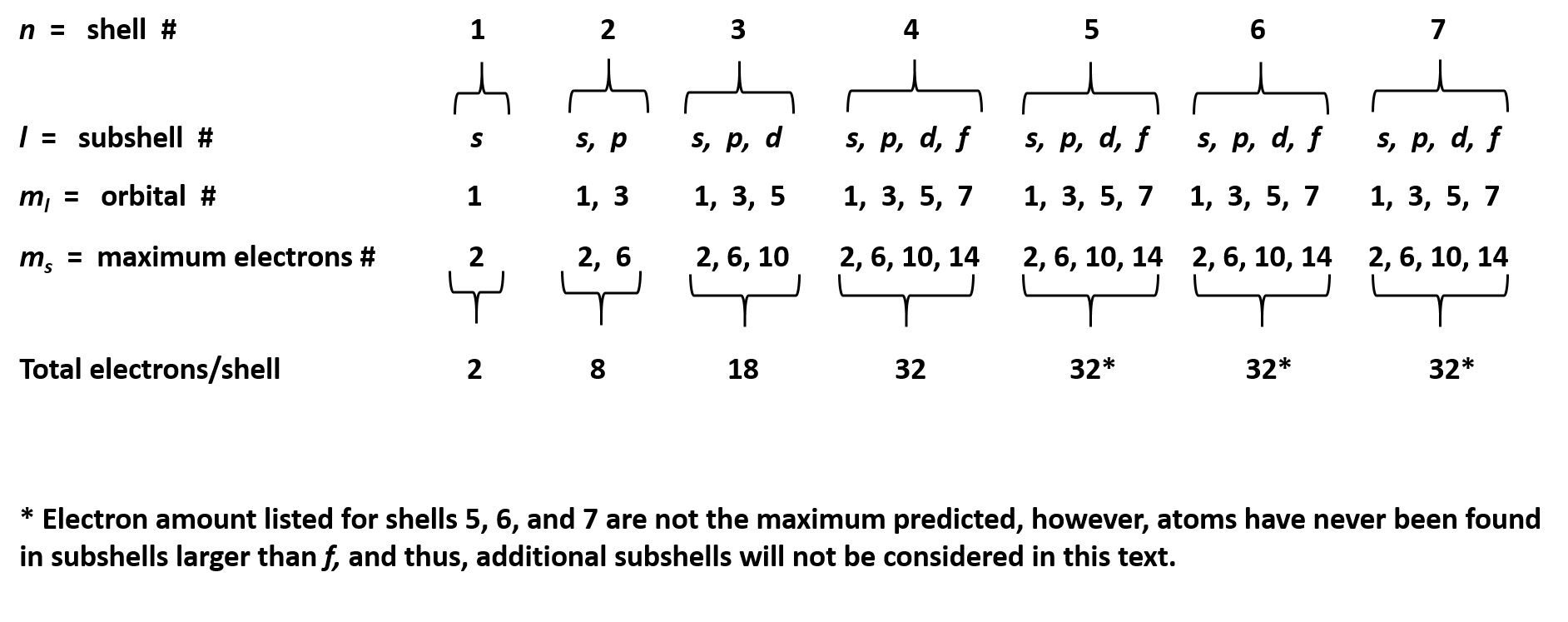
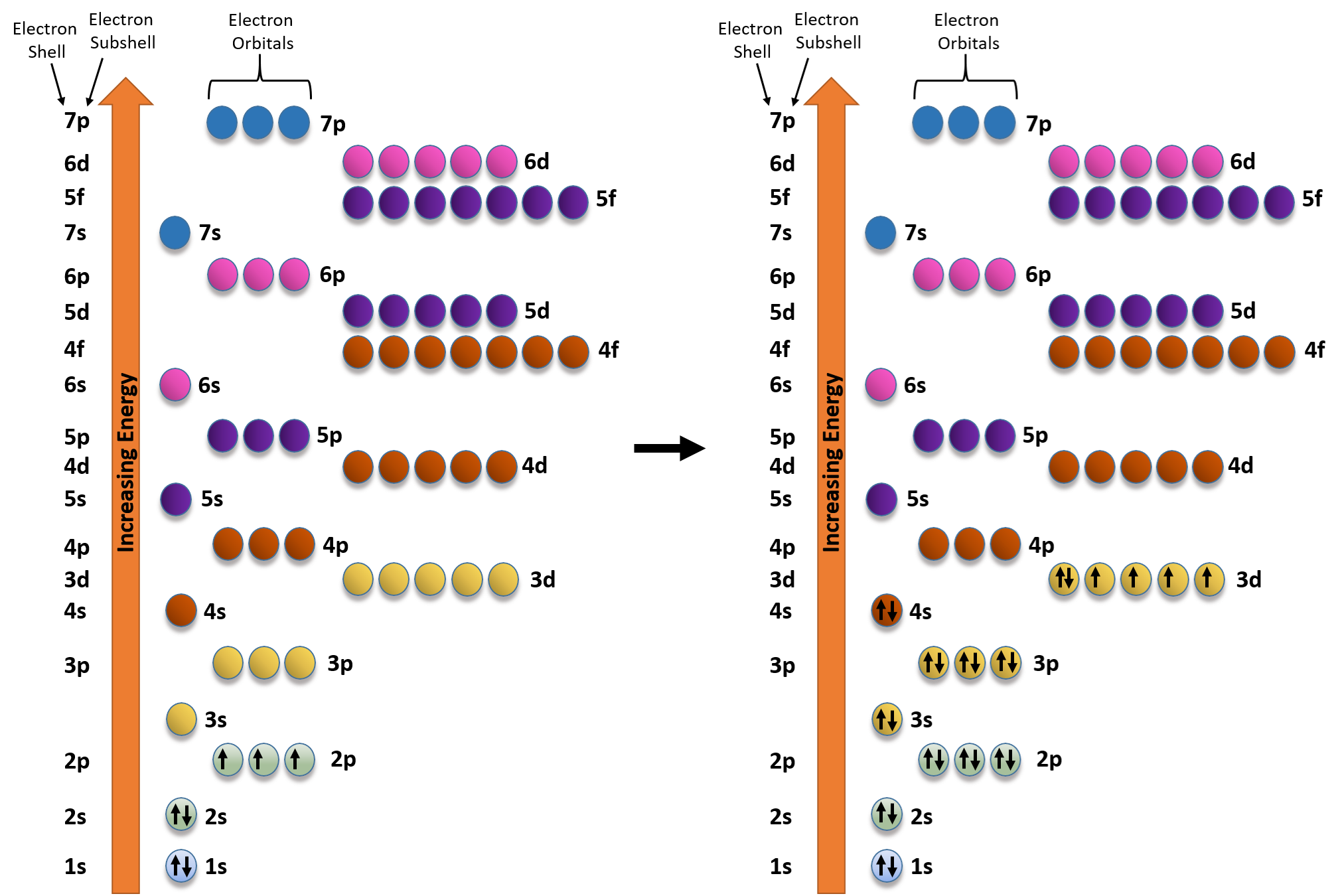
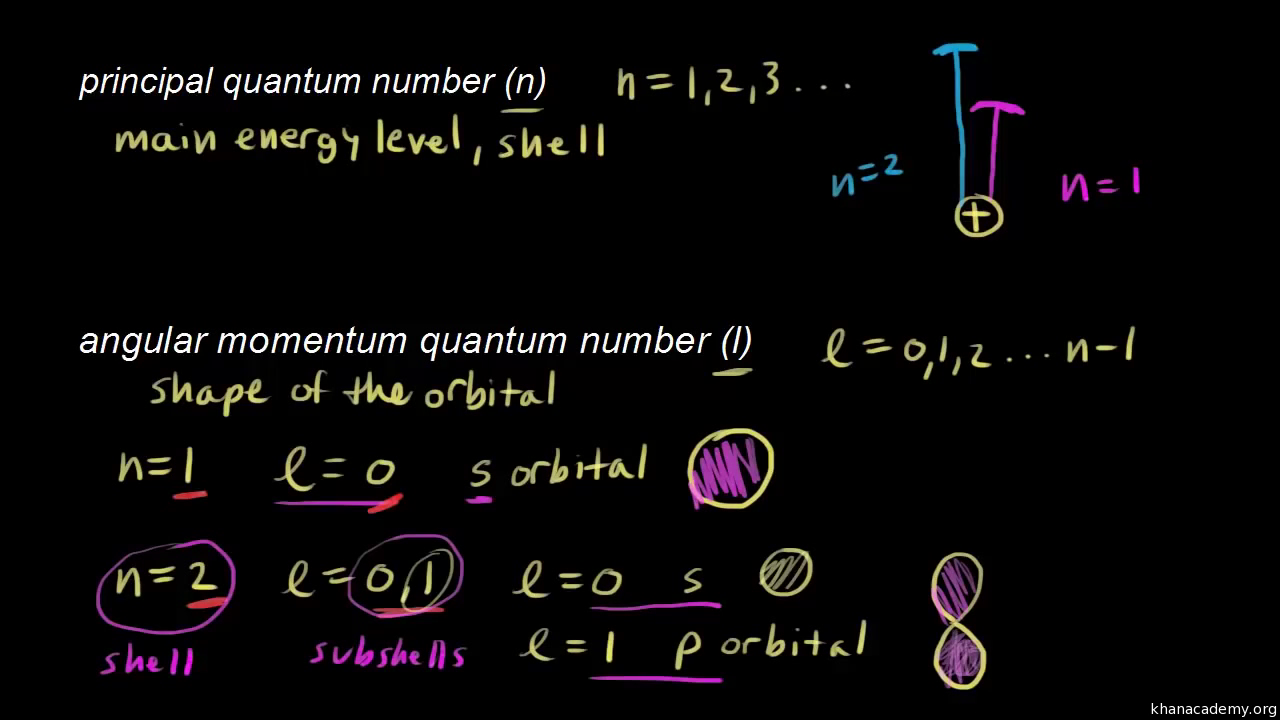
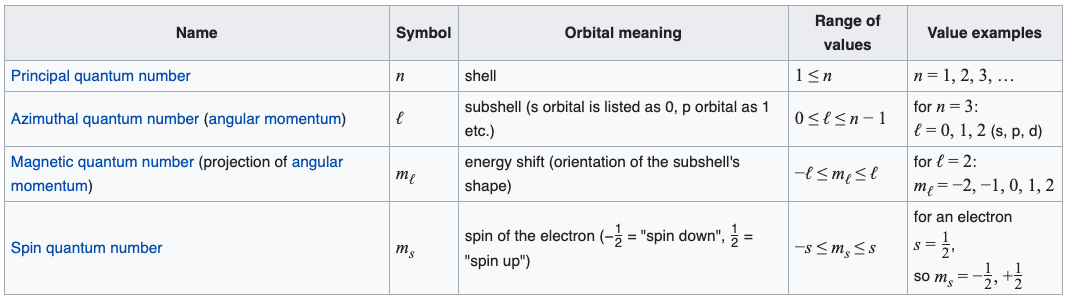
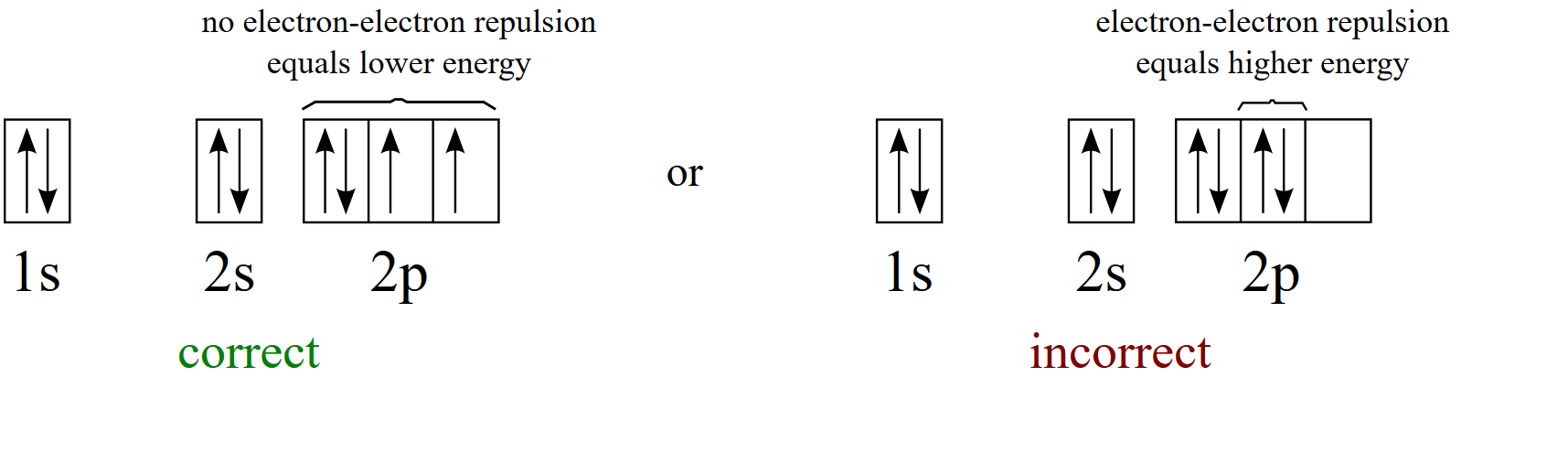
Assuming the electron is in the lowest states and the atom is neutrally charged it will fill an orbital before extending the next one. Principal quantum number n energy level in orbitals and its value could be any positive integer starting from 1 angular momentum quantum number l has to be at least 1 less than n range of values from 0 up to n 1 and each. Which of the following is a correct set of quantum numbers for an electron in a 3d orbital. Each electron in an atom must have its own unique set of quantum numbers is one way to state a the aufbau principle.
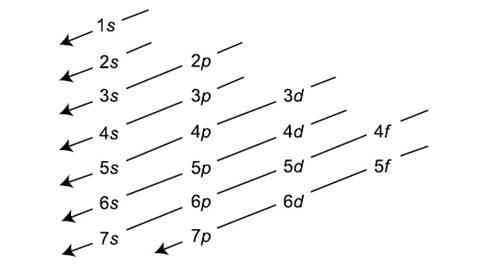
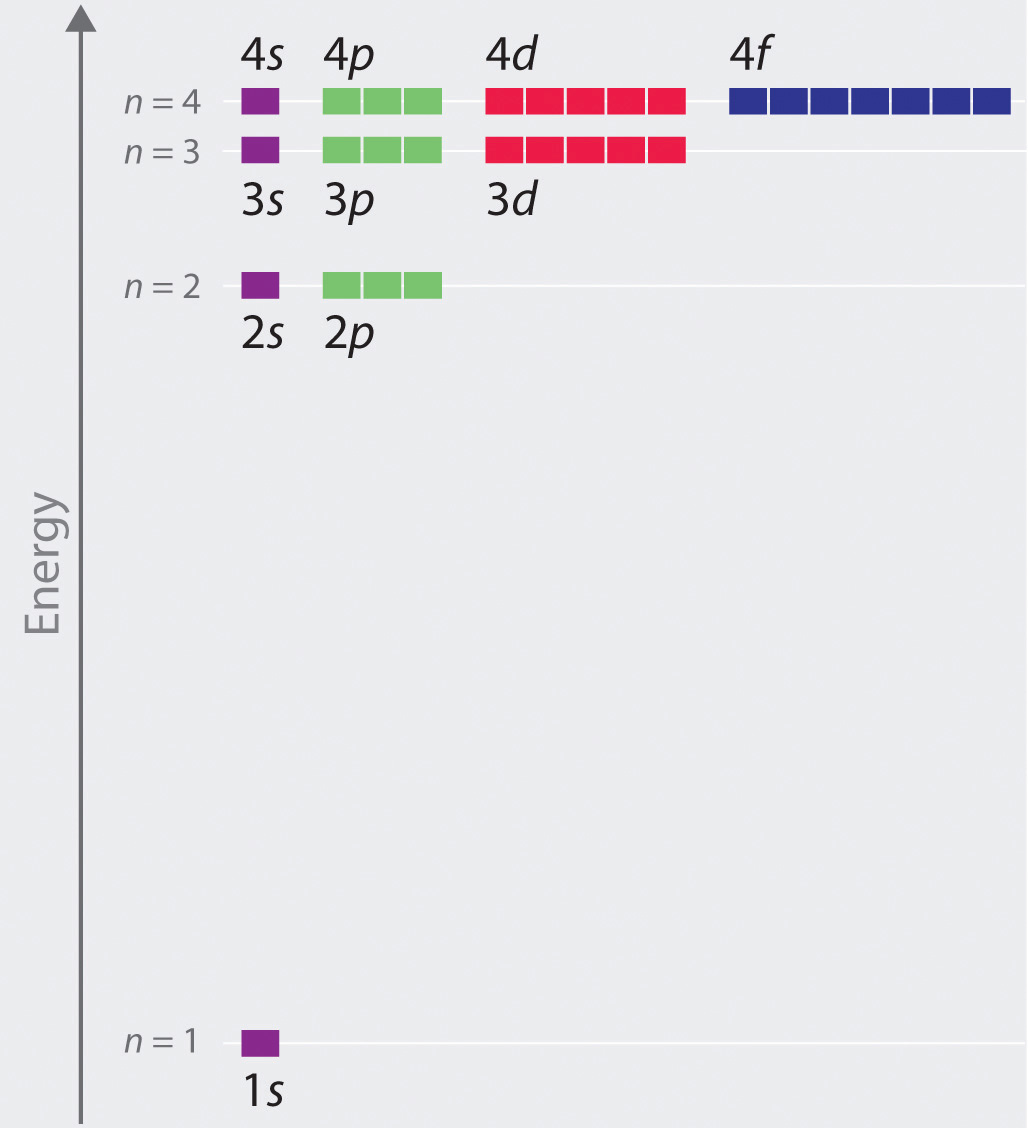
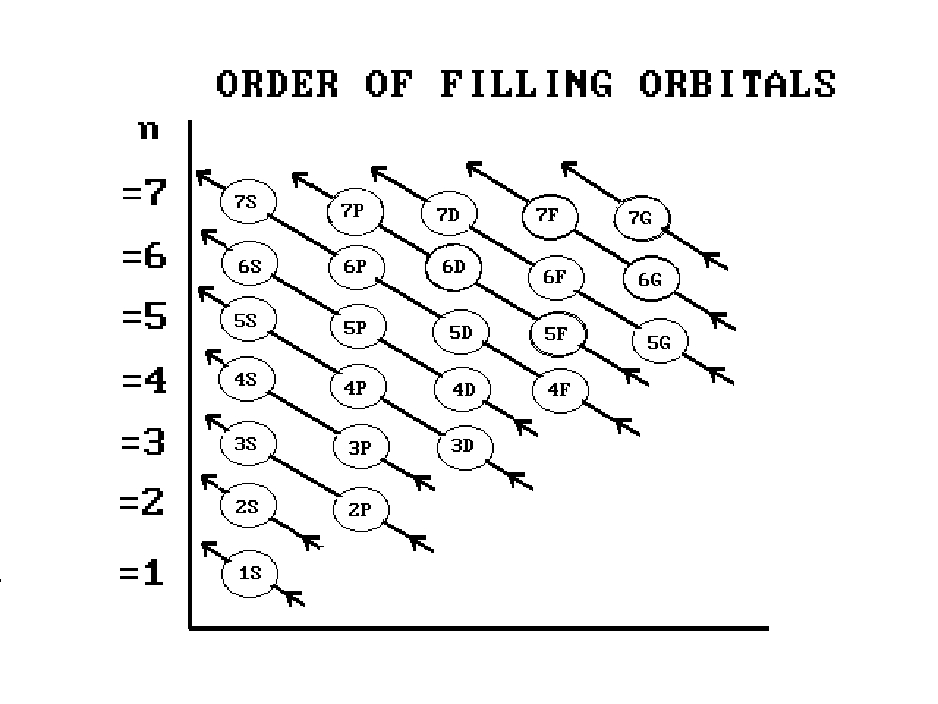
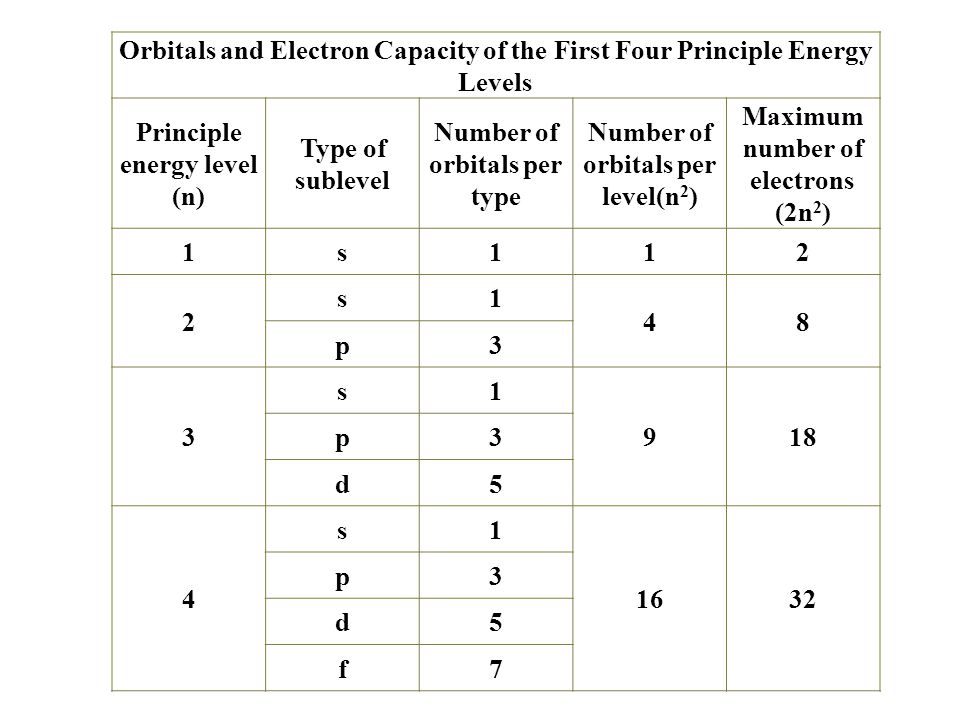
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p. N 4 l 3 m 4 s 12. Which sets of quantum numbers is correct for an electron in 4f orbital. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f.
Which one of the following sets of quantum numbers can correctly represent a 3p orbital. In the quantum mechanical treatment of the hydrogen atom which one of the following combinations of quantum numbers is not allowed. Which of the following sets of quantum numbers nlm1ms refers to a 3d orbital.