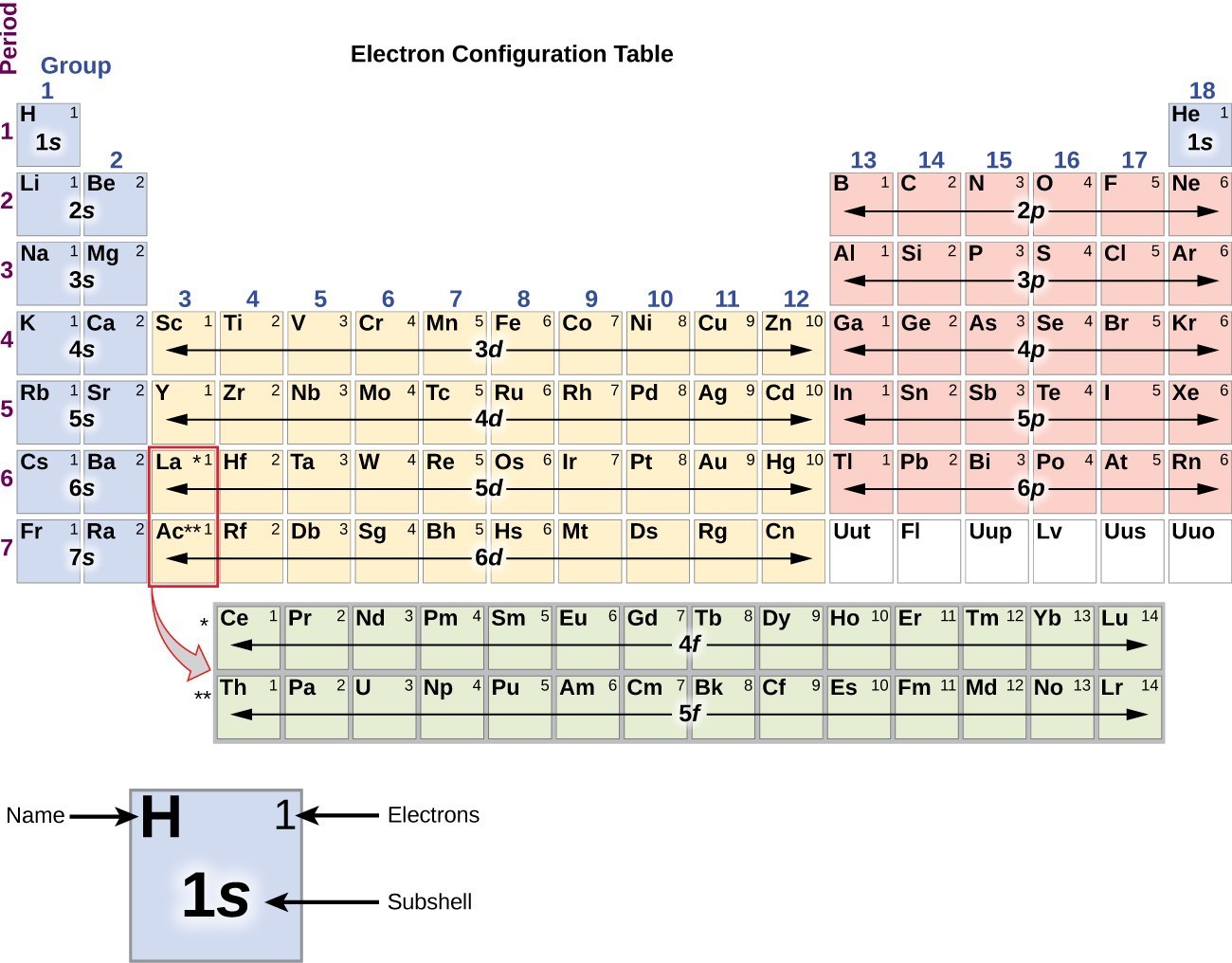
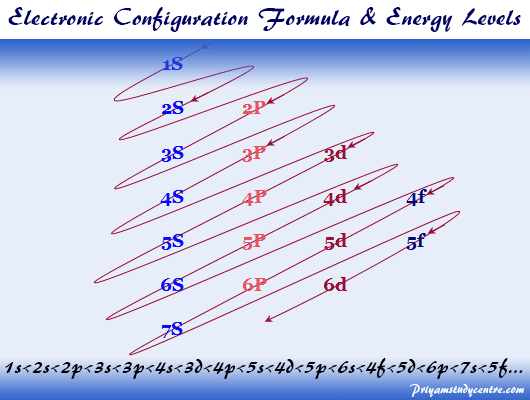
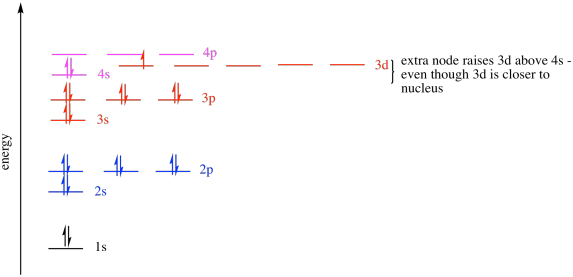
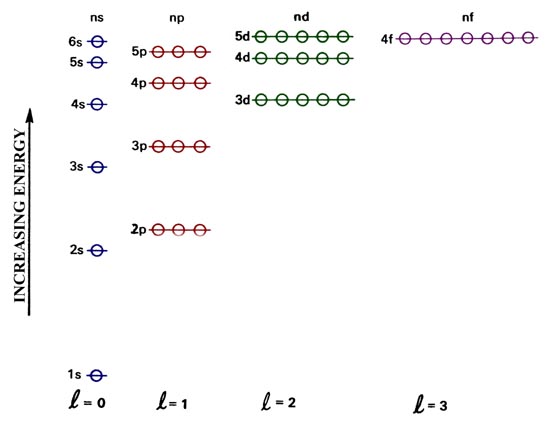
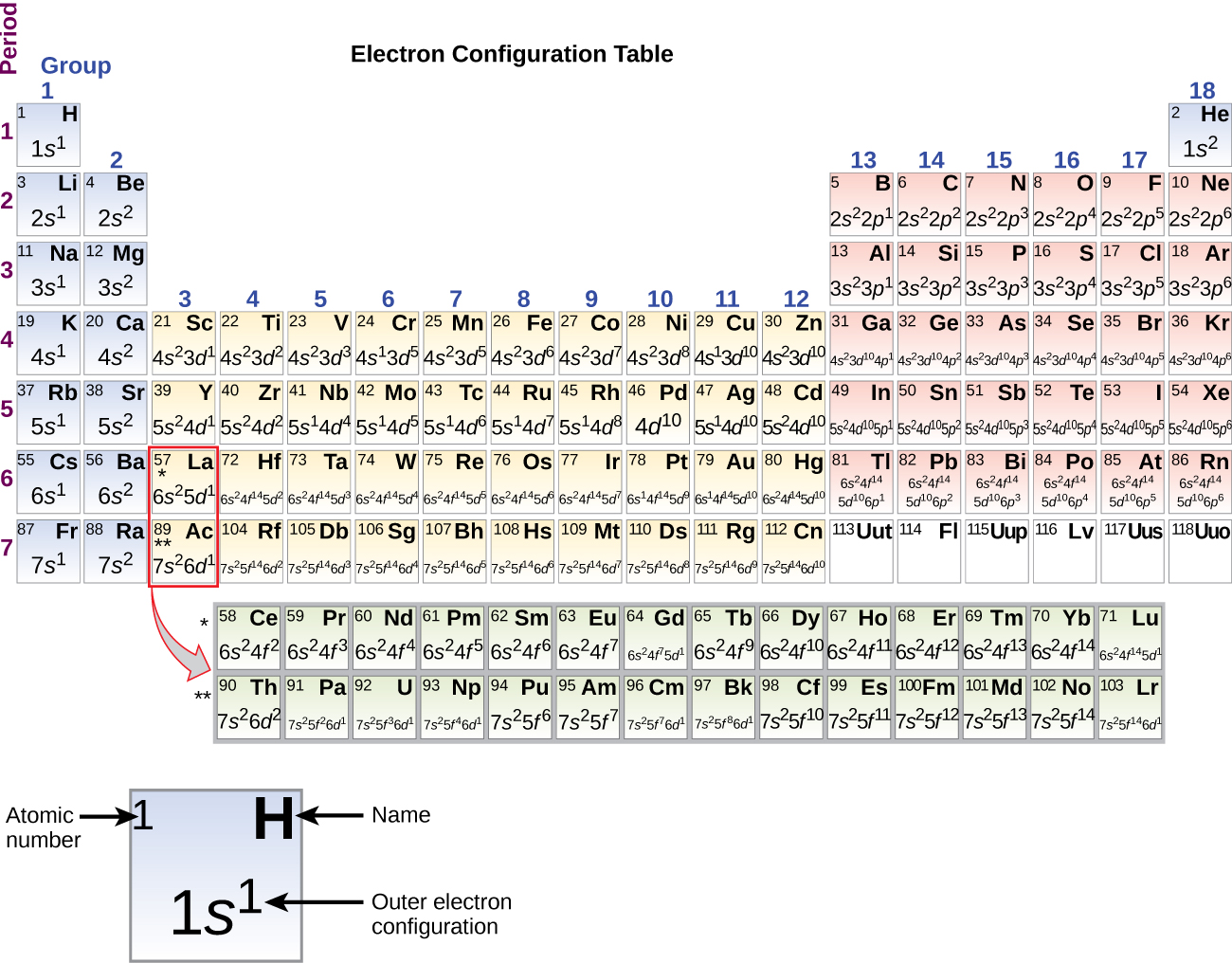
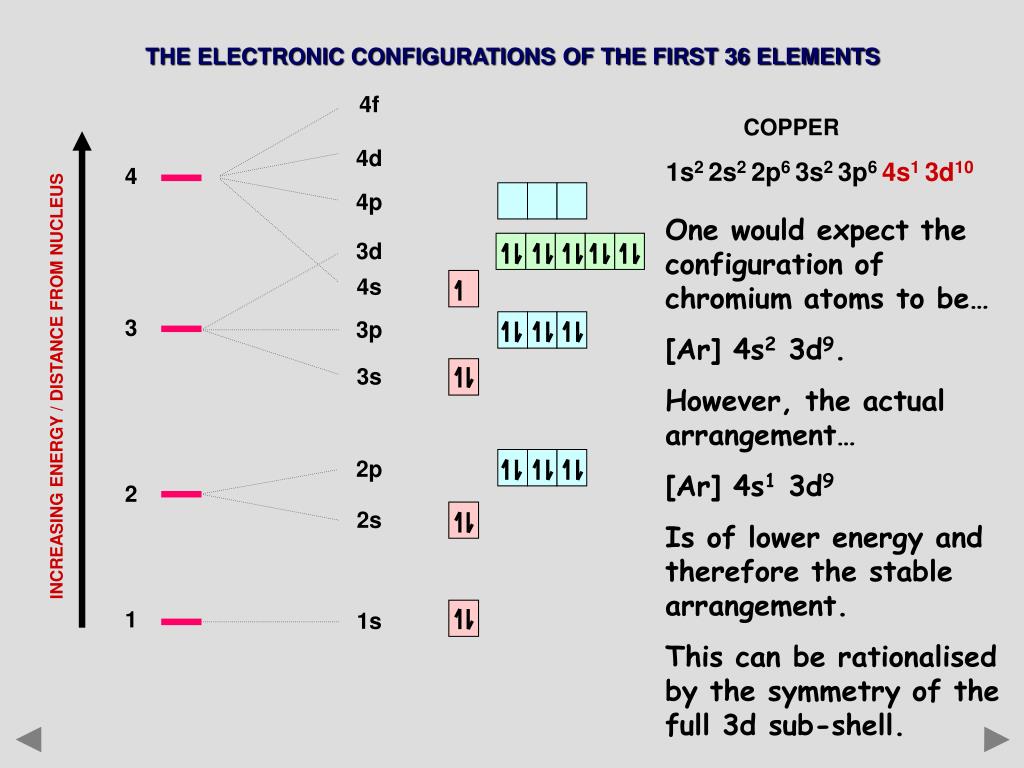
In A Cr Atom A 4s Electron Has Higher Energy Than A 3d Electron
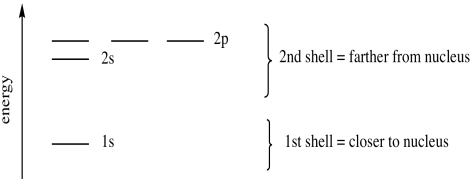
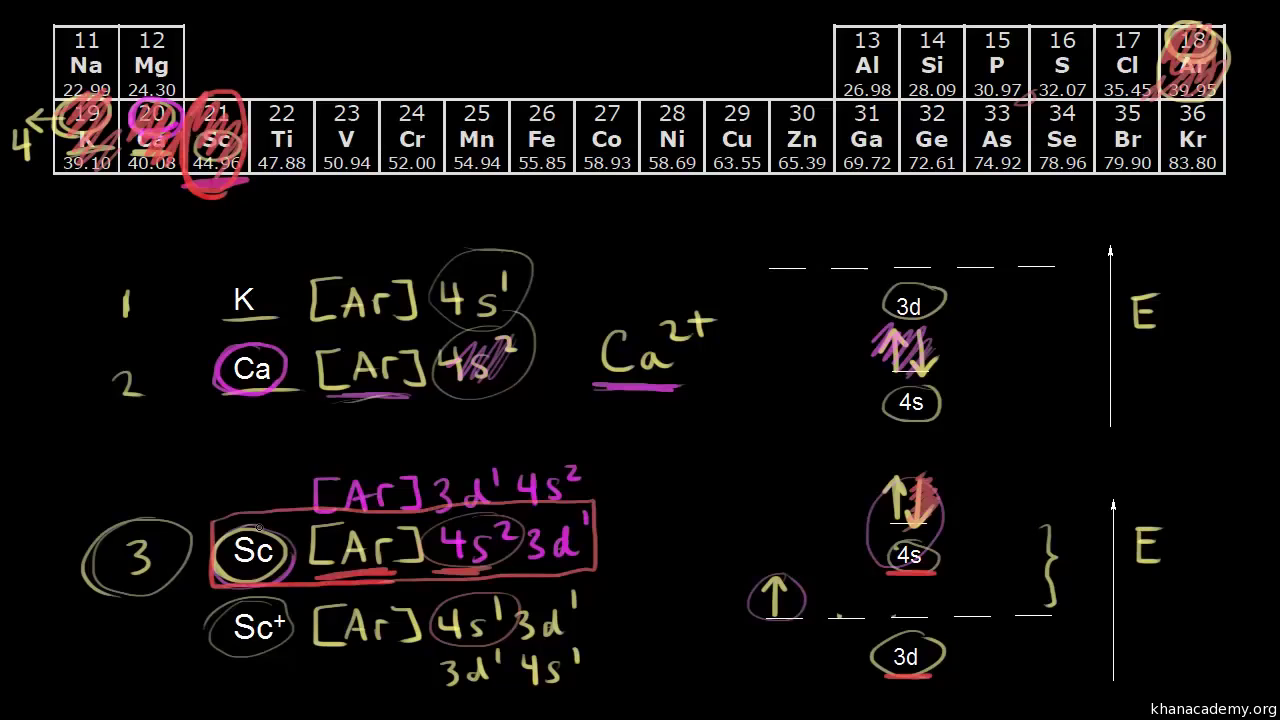
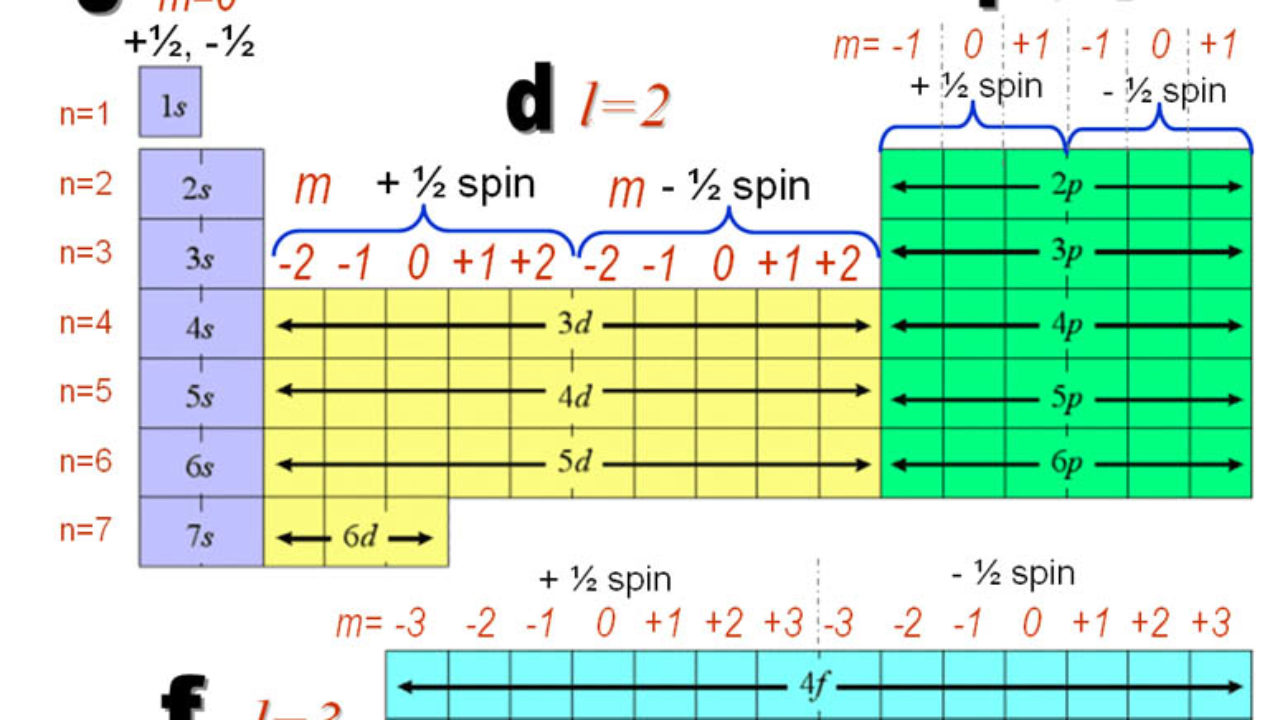
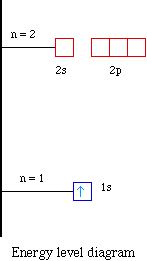
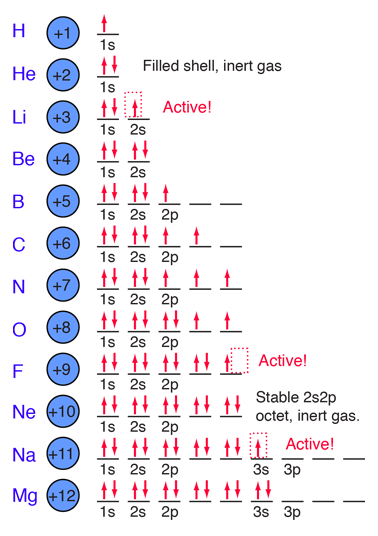
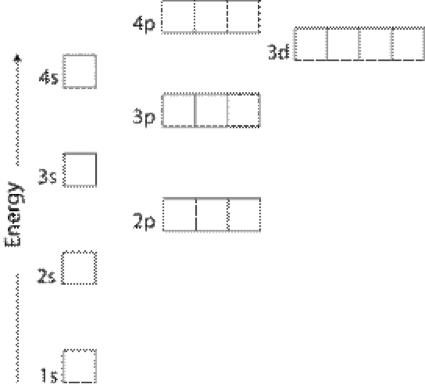
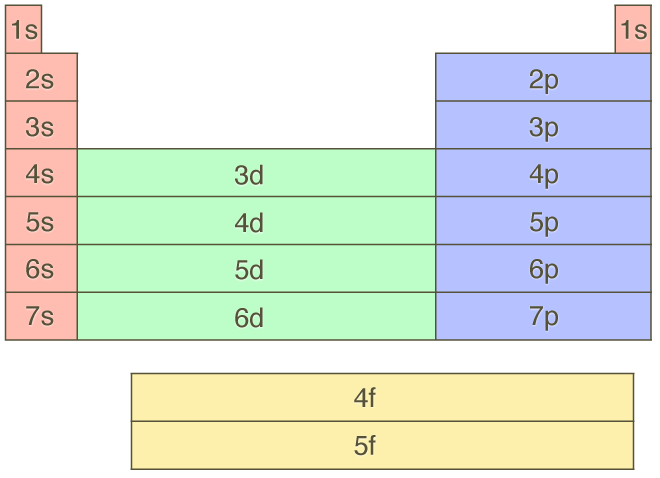
We say that the 4s orbitals have a lower energy than the 3d and so the 4s orbitals are filled first.

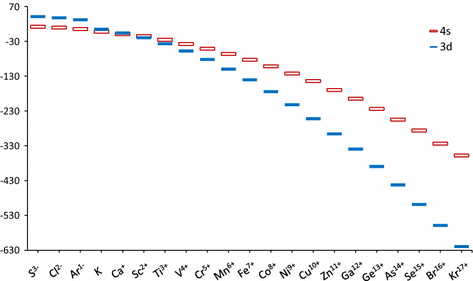
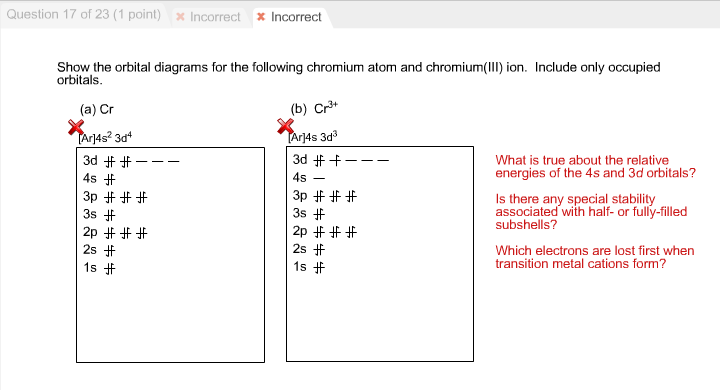
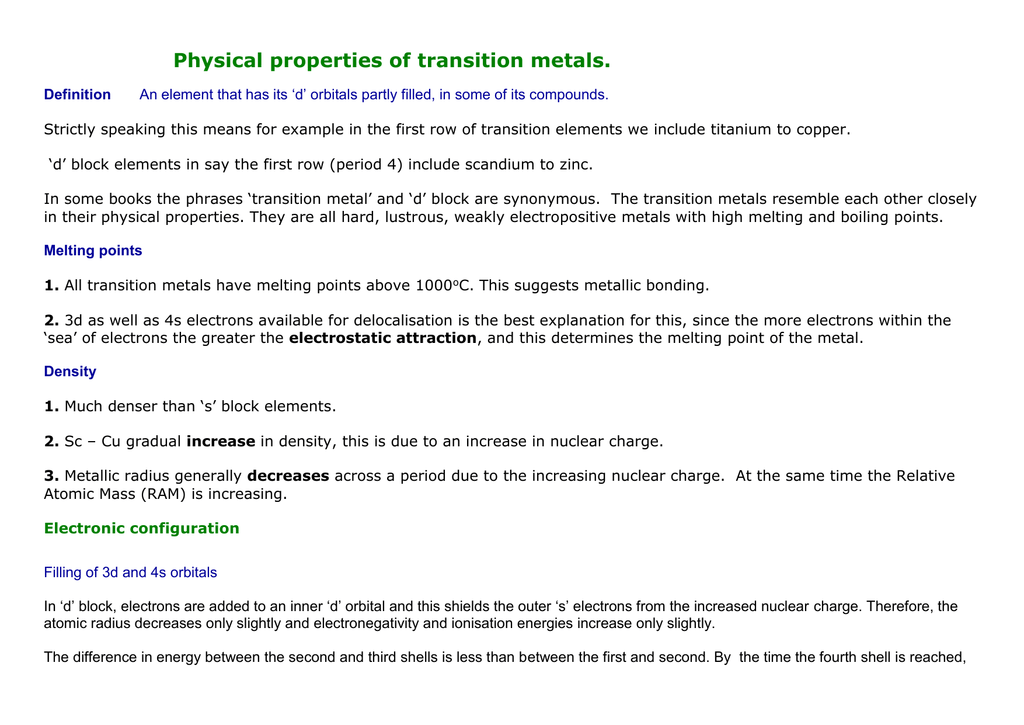
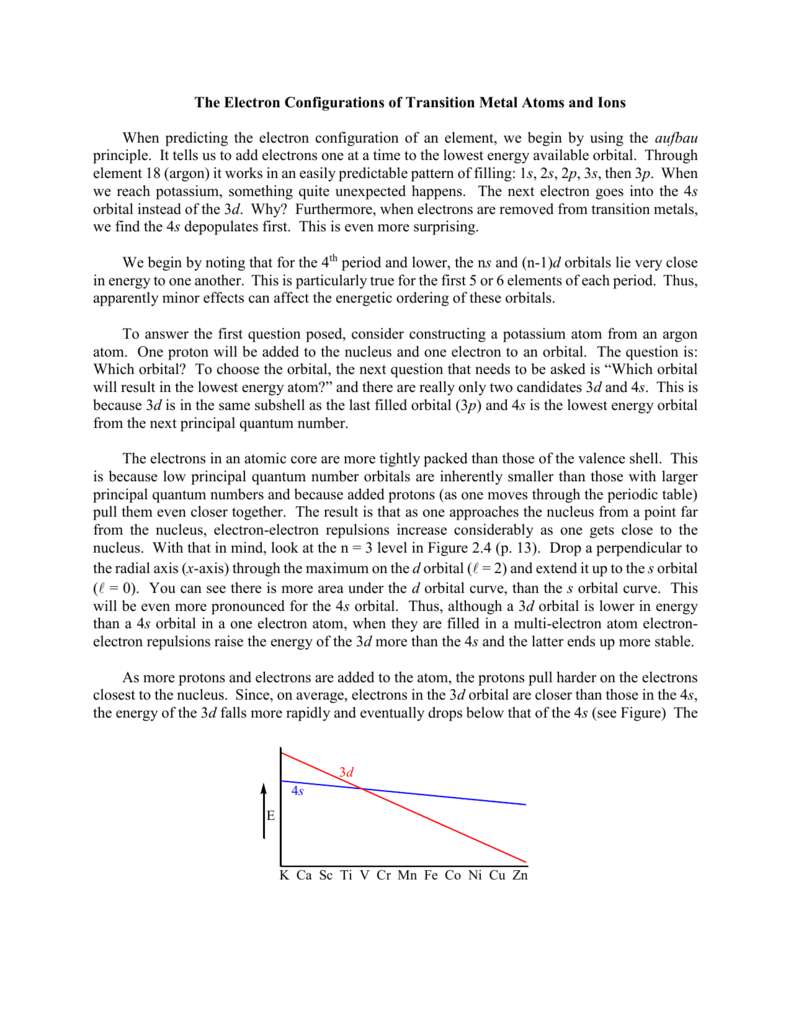
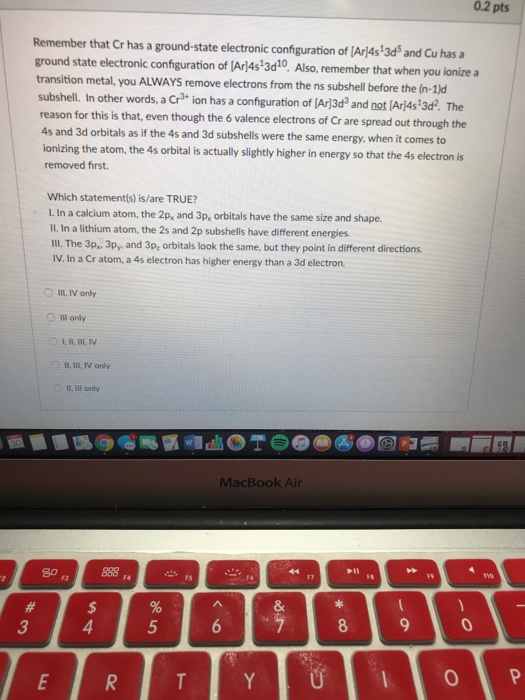
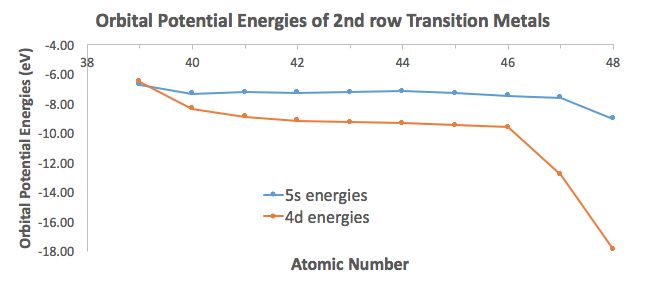
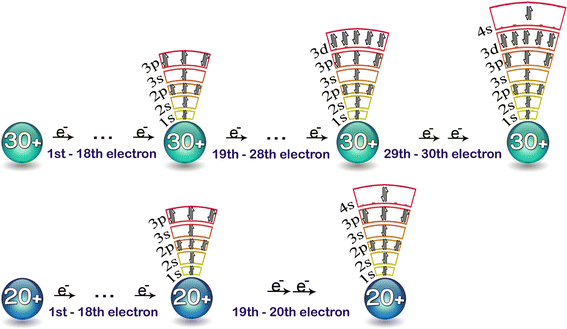
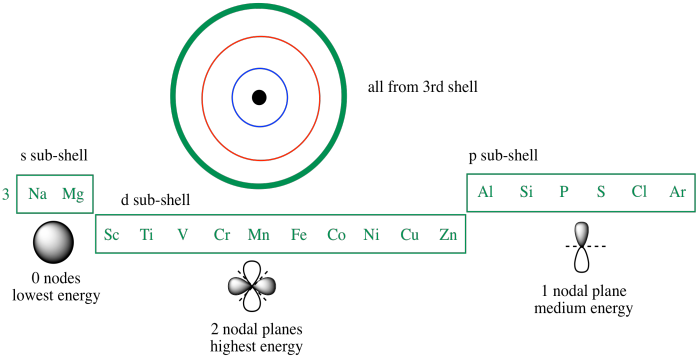
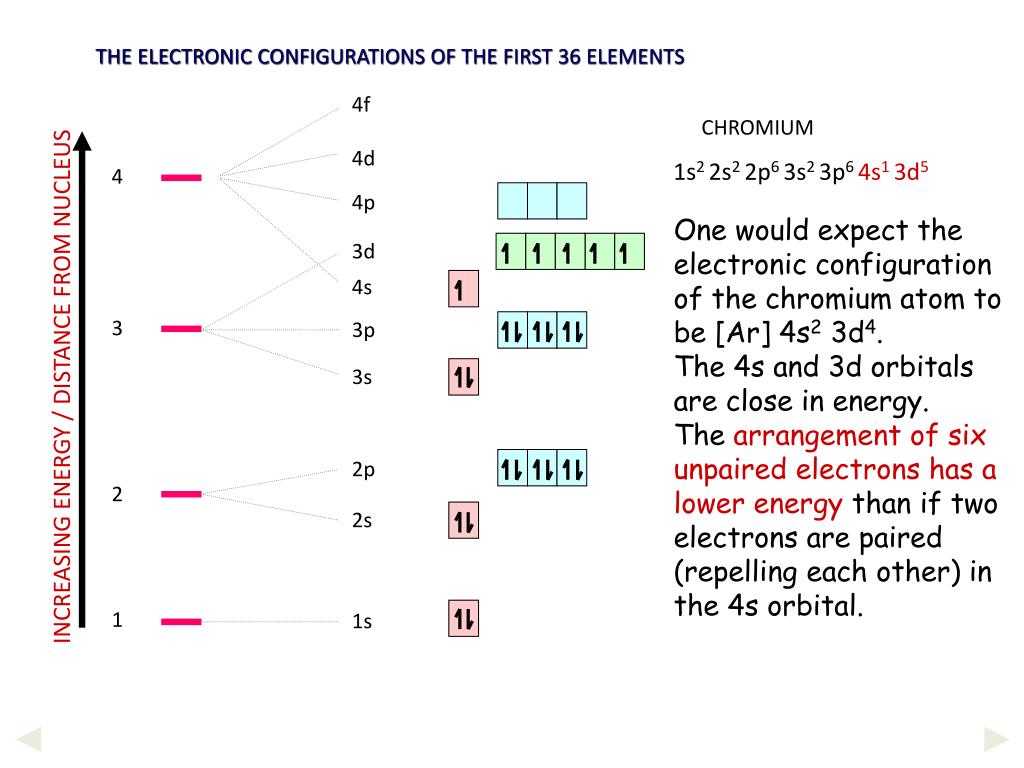
In a cr atom a 4s electron has higher energy than a 3d electron. So this explains why even though we fill the 4s before 3d orbitals we will still ionize 4s electrons before 3d electrons. In a hydrogen atom with just one electron 4s has a higher energy than 3d as you might expect. The electrons lost first will come from the highest energy level furthest from the influence of the nucleus. Once 3d orbitals are occupied by electrons like in the case of transition elements because they are closer to the nucleus they will repel the 4s electrons further away from the nucleus and cause it to have higher energy level.
The 3d orbitals have a slightly higher energy than the 4s orbitals. So the 4s orbital must have a higher energy than the 3d orbitals. Hence electrons are lost from 4s orbital first because electrons lost first will come from the highest energy level furthest away from the nucleus. So the 4s orbital must have a higher energy than the 3d orbitals.
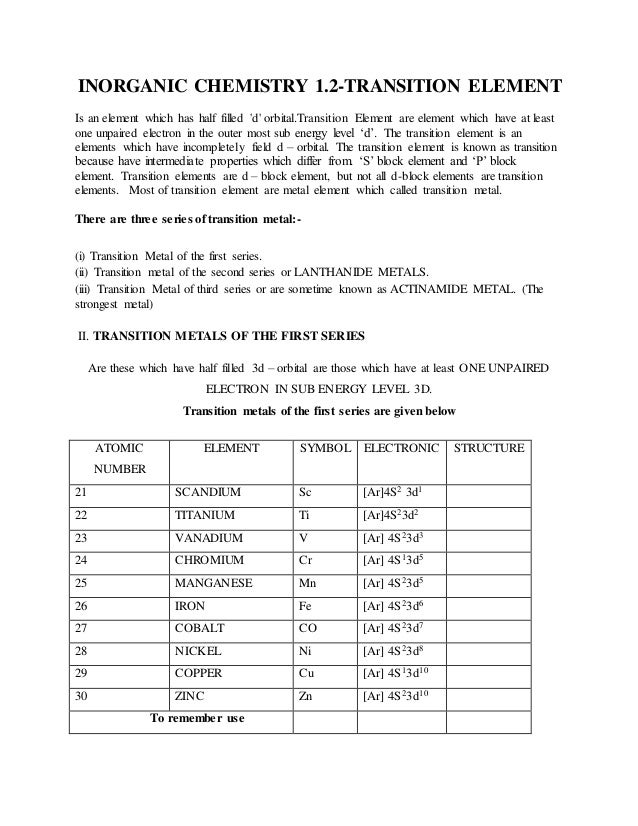
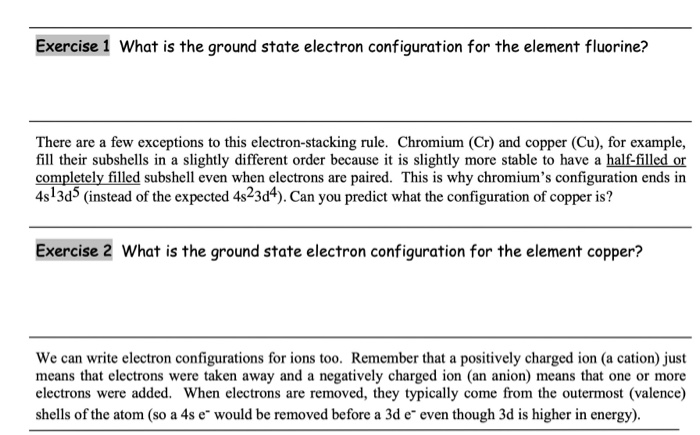
We know that the 4s electrons are lost first during ionisation. We say that the 4s orbitals have a lower energy than the 3d and so the 4s orbitals are filled first. The electrons lost first will come from the highest energy level furthest from the influence of the nucleus. After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4.
When 3d orbitals are filled 4s is no longer lower in energy. So because the 4s orbitals has the lower energy it gets filled first. As more electrons are added because of the. Therefore the expected electron configuration for chromium will be 1s 2 2s 2 2p 6 3s 2 3p 4 4s 2 3d 9.

If 4s Orbitals Are Higher In Energy Than 3d Orbitals Then Why Do Electrons Fill Up In 4s Before Filling Up In 3d Quora
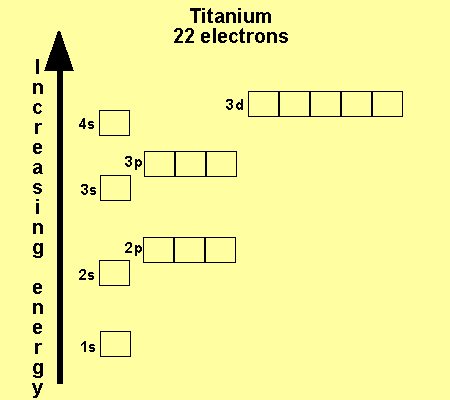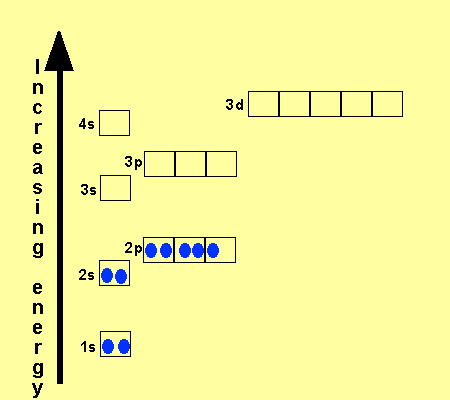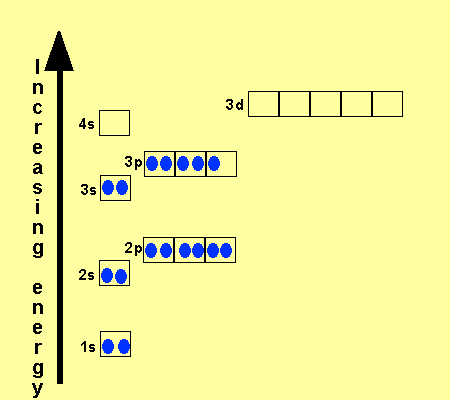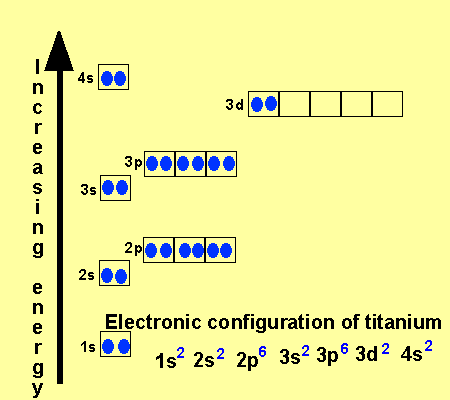





























_and_4s_(color)_Electron_Clouds.jpg)